Pharmacologic Approaches to Maintaining Sinus Rhythm
Except for patients in whom the cause of AF is reversible, pharmacologic therapy likely will be required to maintain sinus rhythm after cardioversion. In approximately 50% of AF patients who undergo cardioversion to sinus rhythm, AF will return within 1 year if prophylactic drug therapy is not employed; AF will recur in approximately 75% of patients within 4 years.24 Before prescribing medication to maintain sinus rhythm, the clinician must assess the patient for underlying cardiovascular disease [see Table 1]. The presence of heart failure, coronary artery disease (CAD), or hypertension with left ventricular hypertrophy has a critical impact on the selection of antiarrhyth-mic medications [see Figure 4].
Class I antiarrhythmics frequently suppress left ventricular function. Randomized clinical trials have demonstrated that amiodarone and dofetilide maintain sinus rhythm without reducing survival in AF patients with heart failure.37-39 As a result, these two drugs have become first-line therapy in this patient subgroup. In patients with ICDs, sotalol may be used safely.40,41
Agents with beta-blocking properties are preferred for patients with CAD. Sotalol has the advantage of blocking both beta-adrenergic receptors and potassium channels. In addition, sotalol has been shown to reduce reinfarction rates after a myo-cardial infarction, and its use has been associated with a trend toward reduced mortality.42 However, in patients with concomitant heart failure or reduced ventricular function, amio-darone or dofetilide is preferable.
Hypertension and left ventricular hypertrophy may affect drug selection. If the left ventricular wall thickness is 14 mm or greater, amiodarone is recommended.
Although these recommendations can be applied to the majority of patients with AF, a number of distinct clinical scenarios require a tailored approach. In patients who do not have structural heart disease but who experience AF during exercise or under adrenergic stimulation, beta blockers are the treatment of choice, followed by sotalol or amiodarone. Vagally mediated AF that is not associated with structural heart disease often responds to disopyramide, a vagolytic medication. Second-line therapy includes flecainide and amiodarone.
Combination therapy may be used when a single medication fails to maintain sinus rhythm. With the combination of medications comes the increased risk of drug-induced side effects, notably, torsade de pointes and heart failure. Monitoring of symptoms and the width of the QTc and QRS intervals is critical.
Monitoring of antiarrhythmic therapy ECG monitoring is necessary in all patients receiving antiarrhythmic medications for maintenance of sinus rhythm. If flecainide or propafenone is used, QRS widening should not exceed 150% of pretreatment QRS width. QRS width should be assessed during exercise ECG testing, typically within 3 days after starting the medication. With all antiarrhythmics except amiodarone, QTc width should not exceed 520 msec. In addition, renal function and levels of serum potassium and serum magnesium should be monitored periodically, because abnormalities in these levels may predispose to arrhythmias.
Outpatient Initiation of Antiarrhythmic Drugs
In a subset of patients with AF, drugs for restoration and maintenance of sinus rhythm can be started safely in the outpatient setting. Advantages of this approach are elimination of the need for DC cardioversion, reduction of hospitalization time, and a decrease in early recurrences of AF after conversion to sinus rhythm. Although outpatient pharmacologic therapy to restore sinus rhythm is appealing, the concern for induction of life-threatening arrhythmias often precludes use of this approach.
Flecainide and propafenone may be initiated on an outpatient basis if the patient has no history of heart failure; if there is no left ventricular dysfunction; if the QRS width is normal; and if the QTc interval is not prolonged. Patients should have both a normal ECG (without any evidence of bradycardia, sinus node disease, or AV nodal disease) and a documented history of at least one episode of inpatient cardioversion with these medications during which no conduction abnormality was unmasked. Amio-darone and sotalol may be started in the ambulatory setting, provided there is no history of structural heart disease, left ventricular hypertrophy, reduced left ventricular function, bradycardia, sinus node or AV nodal conduction disease, hypokalemia, hypo-magnesemia, or previous arrhythmias other than AF or atrial flutter. Flecainide, propafenone, amiodarone, or sotalol should not be started if the patient is also taking other medications that may prolong the QTc interval or predispose to electrolyte abnormalities. Dofetilide, disopyramide, procainamide, and quinidine typically should not be started in the ambulatory setting.1
Nonpharmacologic Approaches to Maintaining Sinus
Rhythm
Several mechanical techniques offer the benefit of reducing the use of antiarrhythmics. The need for anticoagulation with these techniques remains uncertain, however.
Catheter-based ablation Radiofrequency energy emitted from intravascular catheters promotes the generation of endo-cardial scars to eliminate AF. These procedures focus primarily on elimination or isolation of ectopic foci, many of which are located in the pulmonary veins. Although these procedures have the potential to cure AF, many patients experience recurrence of AF. The risks of catheter-based ablation include thromboem-bolism, pulmonary vein stenosis, and cardiac perforation.43
Endovascular radiofrequency ablation is less suited to AF than to atrial flutter, which it can cure with minimal risks and a high rate of success. Ablation of atrial flutter typically involves creating a scar within the right atrium and therefore has a lower risk of complications than AF ablation of the pulmonary veins. Radiofrequency ablation for atrial flutter is curative in more than 90% of cases and should be considered primary therapy for these patients.
Surgical ablation Surgical ablation of AF is similar in concept to catheter-based ablation. During open thoracotomy, linear lesions are created across atrial tissue to generate scars that will act as electromechanical obstacles, extinguishing the reentrant circuits needed for the maintenance of AF. There is a greater than 90% rate of success in eliminating AF with this procedure; however, approximately 25% of patients require a permanent pacemaker for sinus node dysfunction postoperative-ly.4547 This approach has an operative mortality of less than 1% but involves the morbidity of an invasive surgical procedure. The procedure is most often utilized when patients are undergoing cardiac surgery for other indications. The techniques utilized to generate the scars, as well as the location and number of scars created, continue to be modified to reduce surgical time while maintaining efficacy.
Atrial pacing In patients requiring ventricular pacing, the addition of atrial pacing reduces the risk of AF. However, the use of atrial pacing as the primary treatment to prevent AF has not been validated.
Atrial defibrillators Implantable devices to detect and provide DC cardioversion for AF have been shown to successfully terminate AF in more than 95% of episodes.51 Although promising, the use of atrial defibrillators is limited by the generation of pain associated with the release of the electrical shock, as well as the risks associated with device implantation (typically, bleeding and infection). As a result, atrial defibrillators have been used in patients who are unable to tolerate a strategy of ventricular rate control and whose condition is refractory to pharmaco-logic and ablative therapies.
Control of ventricular rate
Ventricular rate control must be addressed both in the acute and the chronic setting. Medication selection in these scenarios is influenced by the rate of onset of the medication, its potential side effects, and its convenience of use.
Hemodynamically unstable patients with angina, myocar-dial infarction, heart failure, or symptomatic hypotension should be considered for acute conversion to sinus rhythm rather than rate control. In contrast, acute rate control can often be achieved rapidly in hemodynamically stable patients through the use of intravenous beta blockers, diltiazem, verap-amil, or digoxin. Oral formulations of these medications are utilized for transition to long-term rate control [see Table 4]. More than one medication is often required to achieve ventricular rate control. Although digoxin is available orally and intravenously, its onset of action is at least 1 hour after infusion, so it is rarely sufficient for stand-alone therapy in the acute clinical setting.
Depending on the clinical scenario, specific agents may be more or less preferable for rate control. This is true of patients with reduced ventricular function, CAD, high sympathetic tone, pulmonary disease, and atrial flutter.
Table 4 Drugs for Ventricular Rate Control in Atrial Fibrillation10
|
Drug |
I.V. Loading Dose |
I.V. Onset |
I.V. Maintenance Dose |
Oral Loading Dose |
Oral Onset |
Oral Maintenance Dose |
Drug Interactions and Precautions |
|
Esmolol* |
0.5 mg/kg over 1 min |
5 min |
5-20 ^g/kg/min |
Available in I.V. form only |
— |
— |
— |
|
Metoprolol* |
2.5-5 mg over 2 min, up to 15 mg |
5 min |
Bolus every 4-6 hr |
Not applicable |
4-6 hr |
50-200 mg daily in divided doses |
— |
|
Propranolol* |
0.15 mg/kg over 1 min, repeat once |
5 min |
Bolus every 4 hr |
Not applicable |
1-1.5 hr |
80-240 mg daily in divided doses |
— |
|
Diltiazem |
0.25 mg/kg over 2 min |
2-7 min |
5-15 mg/hr |
Not applicable |
2-4 hr |
120-360 mg daily in divided doses |
Increases levels of digox-in, quinidine, simva-statin |
|
Verapamil |
75-150 ^g/kg over 2 min |
3-5 min |
Bolus q. 3-6 hr |
Not applicable |
1-2 hr |
120-360 mg daily in divided doses |
Increases levels of digox-in, dofetilide, quini-dine, simvastatin |
|
Digoxin |
0.25 mg q. 2 hr, up to 1.5 mg |
2 hr |
0.125-0.25 mg daily |
0.25 mg q. 2 hr, up to 1.5 mg |
2 hr |
0.125-0.250 mg/day |
Reduce dosing with renal insufficiency; levels increased by amiodarone, propafenone, quini-dine, diltiazem, verap-amil, spironolactone |
|
Amiodarone |
1.2-1.8 g/day until 10 g total |
1-3 wk |
720 mg/day up to 3 wk; limited data on continuous infusion beyond 3 wk |
800 mg/day x 1 wk, 600 mg/day x 1 wk, 400 mg/ day x 4-6 wk |
1-3 wk |
200 mg/day |
Increases levels of digox-in, procainamide, quinidine, and warfarin |
Note: Typical dosing regimens are provided; however, adjustments are necessary based on individual patient characteristics. *Other beta-blocking medications may also be used.
Reduced Ventricular Function
Diltiazem and verapamil can significantly exacerbate left ventricular dysfunction and associated heart failure and so should be avoided in the acute setting. Beta blockers can also have this effect but are preferable for acute rate control. Intravenous esmolol has the advantage of rapid onset and clearance and so may be used to determine whether a patient with left ventricular dysfunction tolerates intravenous beta blockade. However, the large infusion of saline given with esmolol makes long-term intravenous use unattractive for patients with heart failure. If the patient tolerates intravenous esmolol, the clinician should consider changing to another intravenous beta blocker or to oral beta blockade. In addition, digoxin can be utilized in patients with left ventricular dysfunction without concern for exacerbating heart failure. Intravenous amiodarone may also be used in the subacute setting for rate control of patients with AF and reduced ventricular function.
Chronic rate control can be achieved through the oral administration of beta blockers. Bisoprolol, extended-release metopro-lol, and carvedilol improve symptoms and survival in patients with systolic dysfunction and heart failure independent of atrial rhythm.52-54 These medications should be first-line therapy for long-term rate control in these patients. If these medications are not tolerated, oral amiodarone should be considered. In addition, digoxin is effective and well tolerated in heart failure patients with AF.
Coronary Artery Disease
Beta blockers have been shown to reduce mortality in patients with CAD. Because of this additive benefit, beta blockers typically should be selected for CAD patients.
High Sympathetic Tone
The effects of digoxin are attenuated in patients with high sympathetic tone, so this agent rarely provides significant control of heart rate in acute, high sympathetic tone states.
Pulmonary Disease
Patients with asthma can experience significant exacerbation of their lung disease with the use of beta blockers. In these patients, diltiazem and verapamil should be used. Patients with chronic obstructive pulmonary disease without reactive airway disease may or may not tolerate beta blockers. Use of beta blockers in this population should be carefully monitored.
Atrial Flutter
It is often more difficult to achieve ventricular rate control in patients with atrial flutter than in those with AF. If rate control cannot be achieved easily in patients with atrial flutter, radiofre-quency ablation should be reconsidered.
Monitoring Rate Control
Adequacy of rate control should be assessed both with the patient at rest and under stress. The history, physical examination, and ECG provide significant data for this assessment, but Holter monitoring and exercise stress testing also can be used. The ventricular rate should be maintained between 60 and 80 beats/min during rest and 90 to 115 beats/min during moderate exercise.55,56 If rate control cannot be achieved with pharmacologic therapy, AV nodal ablation, combined with permanent pacemaker insertion, should be considered. In addition, permanent pacemaker insertion may be necessary for patients with AF who have labile responses to phar-macologic therapy to avoid episodes of symptomatic bradycardia.
Table 5 Data Collection for Assessment of Thromboembolic Risks and Need for Antithrombotic Therapy in Atrial Fibrillation
|
Characteristic |
Comments |
|
Age |
|
|
Sex |
|
|
History of hypertension |
Patients with medically treated hypertension are considered hypertensive for risk-stratification guidelines |
|
Diabetes mellitus |
Irrespective of control with insulin or oral medications |
|
Coronary artery disease |
|
|
Heart failure |
Past or current |
|
Hyperthyroidism |
Treatment varies depending on whether currently euthyroid |
|
Rheumatic heart disease |
Defined as involving the mitral valve |
|
Previous thromboem-bolism |
Includes strokes, transient ischemic attacks, and other emboli |
|
Prosthetic heart valves |
|
|
LVEF less than 35% |
LVEF-left ventricular ejection fraction
Antithrombotic therapy
AF (including paroxysmal, permanent, and chronic forms) is associated with an increased risk of stroke and other embolic phenomena. The risk of stroke for an individual AF patient varies according to the presence or absence of a number of thromboembolic risk factors. These factors can be garnered from the baseline history, physical examination, laboratory evaluation, ECG, and transthoracic echocardiogram; assessment of these thromboembolic risk factors can serve to guide antithrombotic therapy [see Table 5].
Current ACC/AHA/ESC guidelines for anticoagulation recommend the use of aspirin or warfarin [see Table 6]. Clinical trials have shown that both aspirin and warfarin significantly reduce AF-related strokes in high-risk patients.57-59 Warfarin reduces the risk of stroke by greater than 60%, whereas aspirin reduces stroke risk by 19%. However, the increased benefits of warfarin must be counterbalanced by the increased risk of hem-orrhage.60 Use of lower-intensity warfarin in combination with aspirin provides no additional stroke prevention over aspirin alone, and the combination of full-dose warfarin with aspirin further increases the risk of intracranial hemorrhage.61,62 After warfarin therapy is started, the international normalized ratio (INR) of prothrombin time should be measured at least weekly until stable dosing is reached, and monthly thereafter [see 1:XVIII Venous Thromboembolism].
Atrial Flutter
Although clinical trial data are limited, epidemiologic studies demonstrate that the risk of stroke with atrial flutter, although less than that with AF, remains elevated.63 As a result, use of warfarin and aspirin in atrial flutter should be based on the current AF guidelines.
Elderly Patients
Patients who are 75 years of age or older are at increased risk for both stroke with AF and bleeding with AF anticoagulation.64 As a result of these increased risks, anticoagulation must be tightly monitored in elderly patients, with a goal of maintaining the INR at 2.
Surgical Procedures
Anticoagulation may need to be discontinued in patients scheduled for elective surgical procedures. AF anticoagulation can be discontinued for up to 1 week for surgical procedures in patients without mechanical heart valves. In patients with mechanical valves, the practice has been to discontinue warfarin 1 week before surgery but to maintain anticoagulation with either unfractionated or low-molecular-weight heparin (LMWH). However, current case reports suggest that LMWH may not provide sufficient anticoagulation for patients with mechanical valves, irrespective of concomitant AF.65 Until further data become available, intravenous unfractionated heparin should be utilized.66
Anticoagulation and Cardioversion
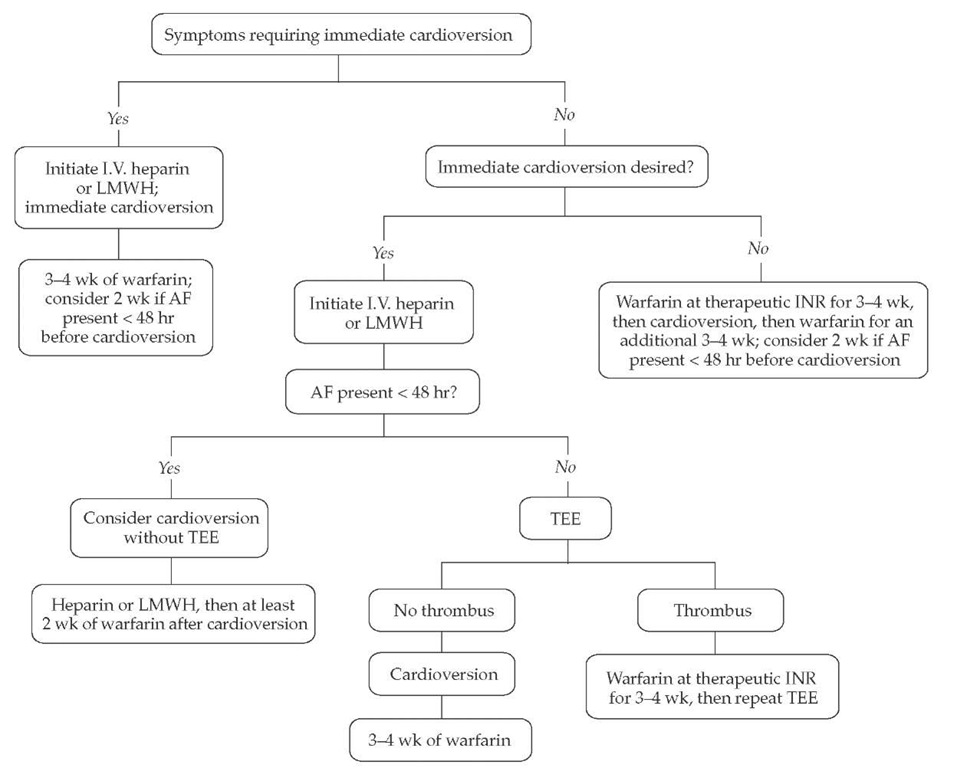
Cardioversion from AF or atrial flutter to sinus rhythm— whether it occurs spontaneously or is accomplished with drugs or electricity—is associated with a 1% to 5% risk of thromboem-bolism. Therefore, strategies for cardioversion of AF should include consideration of anticoagulation; the anticoagulation may start before cardioversion, extend after it, or both [see Figure 5].
If warfarin anticoagulation (to an INR of 2 to 3) is used for 3 to 4 weeks before and after cardioversion, the risk of stroke is reduced to 0.5% in the immediate follow-up period.36,67,68 For that reason, anticoagulation before cardioversion has been strongly advocated.
TEE has been validated as an alternative mechanism to gauge the risk of thromboembolism at the time of cardioversion and immediately afterward. If TEE reveals no evidence of thrombus in the left atrium or left atrial appendage, cardiover-sion can be performed immediately, with a risk of thromboem-bolism comparable to that in patients pretreated with 3 to 4
Table 6 ACC/AHA/ESC Recommendations for Antithrombotic Therapy in Atrial Fibrillation Based on Underlying Risk Factors10
|
Patient Characteristics |
Antithrombotic Therapy |
|
Age < 60 yr, no heart disease (lone atrial fibrillation) |
Aspirin, 325 mg daily, or no therapy |
|
Age < 60 yr, heart disease but no risk factors |
Aspirin, 325 mg daily |
|
Age a 60 yr but no risk factors |
Aspirin, 325 mg daily |
|
Age a 60 yr with DM or CAD |
Warfarin (INR, 2.0-3.0); consider addition of aspirin, 81-162 mg daily |
|
Age a 75 yr, especially in women |
Warfarin (INR, 2.0) |
|
Heart failure |
Warfarin (INR, 2.0) |
|
LVEF s 0.35 |
Warfarin (INR, 2.0-3.0) |
|
Thyrotoxicosis |
Warfarin (INR, 2.0-3.0) |
|
Hypertension |
Warfarin (INR, 2.0-3.0) |
|
Rheumatic heart disease (mitral stenosis) |
Warfarin (INR, 2.5-3.5 or possibly higher) |
|
Prosthetic heart valves |
Warfarin (INR, 2.5-3.5 or possibly higher) |
|
Prior thromboembolism |
Warfarin (INR, 2.5-3.5 or possibly higher) |
|
Persistent atrial thrombus on TEE |
Warfarin (INR, 2.5-3.5 or possibly higher) |
ACC/AHA/ESC—American College of Cardiology/American Heart Association/European Society of Cardiology CAD-coronary artery disease DM—diabetes mellitus INR—international normalized ratio LVEF-left ventricular ejection fraction TEE-transesophageal echocardiography weeks of warfarin therapy.
Figure 5 Cardioversion and anticoagulation strategy for atrial fibrillation. Symptoms that frequently require cardioversion include hypotension, altered mental status, heart failure, pulmonary edema, angina, and myocardial infarction. Adjustment of warfarin intensity and therapy duration is based on individual patient characteristics; the anticoagulation goal is typically an INR of 2-3. (AF—atrial fibrillation; INR—international normalized ratio; LMWH—low-molecular-weight heparin; TEE— transesophageal echocardiography)
This approach allows for immediate cardioversion; however, because cardioversion frequently results in so-called stunning of left atrial and left atrial appendage tissue (a condition that may predispose to thrombus formation), warfarin anticoagulation is required for 3 to 4 weeks after cardioversion, even when TEE performed before car-dioversion showed no thrombus. If TEE does identify thrombus, cardioversion should be postponed for 3 to 4 weeks of anti-coagulation therapy with warfarin, after which TEE should be repeated.
Cardioversion without 3 to 4 weeks of warfarin pretreat-ment and without TEE assessment can be considered if the car-dioversion can be done within 48 hours of the onset of AF or if the patient is started on heparin within 48 hours of AF initiation. Limited data suggest that LMWH may be used instead of intravenous unfractionated heparin, allowing both simplified dosing and transition to warfarin therapy on an outpatient ba-sis.70 This strategy should be most strongly considered in AF patients with significant symptoms of cardiac compromise, including hemodynamic instability, angina, myocardial infarction, heart failure, and shock. The need for anticoagulation after cardioversion in this scenario is unclear, but considering that more than 95% of postcardioversion thromboemboli occur within 10 days after cardioversion, at least 2 weeks of warfarin therapy should be strongly considered if the patient has no contraindications.
Even if heparin was not started until more than 48 hours after the onset of AF, immediate cardioversion also may be necessary if the patient has symptoms of cardiac compromise. Unlike patients who present less than 48 hours after onset of AF, patients with AF of longer duration should receive 3 to 4 weeks of warfarin therapy after cardioversion.
Prolonged anticoagulation after cardioversion should be considered in patients at high risk for both AF recurrence and thromboembolic complications. Atrial flutter is associated with a risk of thromboembolism in the setting of elective cardioversion and should be treated in the same manner as AF.67
Ximelagatran
Ximelagatran is a direct thrombin inhibitor that can be administered orally. In the Sport Prevention Using Oral Thrombin Inhibitor in Atrial Fibrillation-III (SPORTIF III) trial, which was an open-label comparison of adjusted-dose warfarin and fixed-dose ximelagatran, there were no significant differences in rates of stroke, systemic thromboembolism, bleeding, or death with the two drugs.72 The SPORTIF V trial will compare the two drugs in a double-blinded format. Ximelagatran offers a wider therapeutic window than warfarin and requires no monitoring with coagulation studies. If the equivalence of ximelagatran with warfarin is confirmed, the ease of use of this medication will likely lead to its replacing warfarin for many patients with AF. Future studies will be required to determine whether xime-lagatran can be applied to specific settings in which warfarin therapy has been validated.
Treatment in specific clinical scenarios
Cardiac Surgery
AF occurs after 25% of all coronary artery bypass surgeries and after more than 60% of combined coronary artery bypass and mitral valve surgeries.73 Additional risk factors in these cases included advanced age, male sex, preoperative atrial arrhythmias, left atrial enlargement, chronic lung disease, and previous cardiac surgery.74 AF after cardiac surgery leads to a significant increase in length of hospital stay and cost.75 A number of prophylactic therapies to prevent postoperative AF have been examined and validated, including use of beta blockers, sotalol, amiodarone, and postoperative temporary atrial pacing.76 The incremental cost of prophylactic therapy must be balanced against the potential savings achieved by reducing length of stay if AF is prevented. Unless contraindicated, beta blockers should be given to all patients scheduled for cardiac surgery. Sotalol, amiodarone, and biatrial pacing should be considered if patients are at high risk for postoperative AF because of additional risk factors.
Anticoagulation should be given if AF occurs after cardiac surgery and lasts longer than 48 hours. Although sinus rhythm returns spontaneously within 6 weeks in 95% of patients with postoperative AF, pharmacologic or DC cardioversion is often performed, particularly in patients who are symptomatic or he-modynamically unstable.77 Medications to maintain sinus rhythm or to achieve ventricular rate control can be selected on the basis of patient characteristics.
Acute Myocardial Infarction
In patients with acute myocardial infarction, AF is an independent predictor of mortality and stroke. Immediate DC car-dioversion should be performed in patients with severe hemo-dynamic compromise or persistent ischemia. If rate control is possible, digoxin can be combined with a beta blocker if left ventricular function is preserved. Because of the thromboem-bolic risk, heparin should be given acutely and followed with warfarin if AF persists or significant left ventricular dysfunction develops.
Wolff-Parkinson-White Syndrome
Wolff-Parkinson-White syndrome (WPW) in association with AF can be a life-threatening condition. The bypass tract of WPW may allow rapid conduction of atrial activity to the ventricles, precipitating hemodynamic compromise or ventricular fibrillation. In the acute setting, DC cardioversion should be pursued if hemodynamic compromise is present. If the patient is hemody-namically stable, the clinician may consider pharmacologic car-dioversion to sinus rhythm with intravenous procainamide or ibutilide.78 Agents that slow AV conduction are contraindicated, including digoxin, diltiazem, and verapamil. Beta blockers should be used rarely and with extreme caution. Once stabilization is achieved, catheter ablation of the WPW bypass tract should be pursued in all symptomatic WPW patients with AF.
Hyperthyroidism
Hyperthyroidism may cause AF and is associated with an increased risk of stroke. Hence, these patients require anticoagula-tion. Rate control should be attempted with beta blockers, supplemented with diltiazem, verapamil, or digoxin as needed. Warfarin should be given while the patient is thyrotoxic. Once the euthyroid state has returned, use of aspirin or warfarin should be based on underlying risk factors.
Hypertrophic Cardiomyopathy
AF in patients with hypertrophic cardiomyopathy is associated with a high risk of death and stroke.79 Warfarin therapy is recommended (INR, 2 to 3).
Pulmonary Disease
In patients with pulmonary disease, hypoxia and other metabolic disturbances frequently initiate AF. Initial therapy focuses on treating the underlying lung disease. Beta blockers, pro-pafenone, sotalol, and adenosine are contraindicated in patients with reactive airway disease. Diltiazem or verapamil, with or without digoxin, should be utilized for rate control in these patients.