Antigens
An antigen is any substance capable of generating an immune response; that is, antigens react with T cells and B cells to induce the formation of antibodies and to sensitize lymphocytes, and those antibodies and sensitized cells then react with the antigens. The first antigens to be studied were microorganisms and foreign proteins, and it remains true that foreign proteins are almost universally antigenic. In addition, polysaccharides can induce antibody formation when coupled to proteins, and certain purified polysaccharides are themselves effective antigens. One such example is purified pneumococcal polysaccharide, which can be used as a vaccine against the particular strain of pneumococcus from which the polysaccharide is obtained. Although most antigens are macromolecules, some small molecules can also be anti-genic. Antibodies to DNA or RNA occur in many patients with rheumatic diseases, especially systemic lupus erythematosus.
Antigen Recognition
Antigens are recognized not only by antibodies but also by antigen-specific B cell receptors (BCRs) [see B cell receptors, below] and T cell receptors (TCRs) [see T Cell Receptors, below], which are located on the surfaces of B cells and T cells, respectively. In general, TCRs and antibodies recognize different anti-genic determinants.
Two distinct types of TCRs exist: TCR-a| and TCR-y6. Each has different mechanisms for recognizing antigens [see 6:IV Cell-Cell Interactions, Cytokines, and Chemokines in Immune Response Mechanisms]. For example, T cells bearing TCR-a| recognize antigens that have been processed by antigen-presenting cells (APCs) to become peptide fragments bound to major histocom-patibility complex (MHC) class I or class II molecules on the surface of the APCs. In contrast, T cells bearing TCR-y6 appear not to require antigen presentation by MHC molecules. Helper T cells recognize only peptide fragments bound to MHC class II molecules, whereas cytotoxic T cells recognize processed viral antigens presented by both MHC class I and MHC class II molecules on the surface of virus-infected cells. Pure lipids from my-cobacteria can be presented as antigen to TCR-a| by CD1 molecules rather than by MHC class I or class II molecules.
Adjuvant
Adjuvants are substances capable of increasing the immuno-genicity of antigens and are critical in the production of vaccines. Many microbial products have been used as adjuvants,1 including substances from Mycobacterium tuberculosis, bacillus Cal-mette-Guerin (BCG), Corynebacterium parvum, Brucella abortus, and Bordetella pertussis, as well as toxoids from Vibrio cholerae and Clostridium tetani. Adjuvants have also been derived from vaccinia virus and other poxviruses, BCG, and Salmonella by trans-fecting the organism with genes for an antigen of interest.
Others have come from lipopolysaccharide derivatives, such as monophosphoryl lipid A. Freund complete adjuvant, which consists of dead mycobacteria in oil emulsified with an antigen in aqueous solution, is now infrequently used because it generally causes a strong local inflammatory reaction. Other adjuvants are extracts from the soap bark tree (Quillaja saponaria), polymers (e.g., inulin), peptide complexes, and a number of cytokines. The aluminum salt alum is approved for general use as an adjuvant in humans. The complex of antigen and complement fragments, particularly those derived from C3, probably serves as the physiologic adjuvant.
Antibodies
Antibodies are a heterogeneous group of serum proteins called immunoglobulins. Immunoglobulins are secreted by differentiated B cells called plasma cells. According to Sir Francis MacFarlane Burnett’s clonal selection theory, a single plasma cell produces only one specific antibody. This theory is aptly illustrated by the disease multiple myeloma, in which malignant proliferation of plasma cells occurs in bone marrow. The multiple myeloma plasma cell produces an abnormal monoclonal im-munoglobulin called a myeloma protein. Its homogeneity is clearly visible on electrophoresis as a single dense band. Certain antibodies that are produced in response to highly homogeneous antigens, such as streptococcal polysaccharide, may also be relatively homogeneous.
Immunoglobulin molecules consist of two identical heavy chains and two identical light chains [see Figure 1a]. Each light chain is attached to a heavy chain by disulfide (S—S) bonds, and the heavy chains are also attached to each other by one or more S—S bridges. Amino acid sequences in both heavy and light chains are divided into regions that are either constant or variable [see Figure 1b]; in addition, each variable region contains sequences that are hypervariable.
Classification of Immunoglobulins
There are five classes of immunoglobulins, and each class contains a specific heavy chain: IgG contains two y chains; IgM, two ^ chains; IgA, two a chains; IgD, two 6 chains; and IgE, two e chains. There are also two types of light chains, k and 6, which can be differentiated antigenically. One form of IgM, the secreted form, is a pentamer. The monomeric form of IgM is expressed on the extracellular surface of B cells. IgA exists as a monomer or a dimer. The polymeric forms of IgM and IgA have an additional J (joining) chain that facilitates polymerization.
IgG IgG is the major immunoglobulin in the serum, where it exists as a monomer. IgG has a half-life in the blood of approximately 23 days. It is the main antibody raised after antigenic challenge. There are four subclasses of IgG—IgG1, IgG2, IgG3, and IgG4—each different in structure and biologic properties. For example, only IgG1 and IgG3 bind the first component of complement and adhere to monocytes. The antibodies that coat microorganisms and render them more susceptible to phagocytosis (i.e., opsonization) are of the IgG class. IgG antibodies can also neutralize viruses and toxins such as diphtheria toxin. Human antibodies to polysaccharides are mainly of the IgG class, but lesser amounts of IgM and IgA are also produced. Although the fetus does not produce this class of immunoglobulin, IgG readily crosses the placenta; therefore, IgG antibodies found in the newborn are from the mother.
Figure 1 (a) The immunoglobulin molecule is a Y-shaped protein made up of four polypeptide chains. Two heavy chains (blue) are joined to two light chains (green) by disulfide bonds. Blue squares represent intrachain S—S bonds; blue bars indicate interchain S—S bonds. The heavy chains extend from the stem of the Y into the arm; the two light chains are confined to the arms. Each polypeptide has regions whose amino acid sequences are constant (white and yellow) and variable (red). The variable regions also contain hypervariable regions. All antibodies of a given type have the same constant regions, but the variable regions differ from one clone of a B cell to another. The heavy- and light-chain variable regions fold to create an antigen-binding site. (b) Schematic model of the domain structure of an antibody molecule. The domains have a characteristic folding pattern, which is also seen in the T cell receptor and proteins of the major histocompatibility complex.
Clinically, IgG has been used successfully for reconstituting the immunity of patients with primary immune deficiencies, such as agammaglobulinemia, and for preventing hemolytic disease in the newborn. Women with Rh-negative blood who bear a fetus with Rh1-positive red blood cells are sensitized at the first delivery by Rh1-positive red blood cells from the fetus. The mother then produces anti-Rh1 IgG antibodies that will cross the placenta during subsequent pregnancies; these antibodies react with fetal red blood cells, causing hemolytic disease. Erythro-blastosis fetalis can be prevented by injecting IgG rich in anti-Rh-positive antibodies (RhoGAM) into an Rh1-negative mother at the time of delivery or abortion. These antibodies presumably combine with any fetal Rhl-positive red blood cells present and prevent them from immunizing the mother.
IgG can be split into three fragments by the proteolytic enzyme papain. Two of the fragments are similar and are called Fab; the third is called Fc [see Figure 1a]. The Fc portion is responsible for the biologic activity of the various immunoglobulins; among other things, the Fc portion controls the ability of immunoglobulins to bind to cells, fix complement, and traverse the placenta. Another proteolytic enzyme, pepsin, splits the IgG molecule behind the S—S bonds that bridge the heavy chains, leaving one large fragment, F(ab’)2, which is able to bind and precipitate antigen because of its bivalency and capacity to form a lattice.
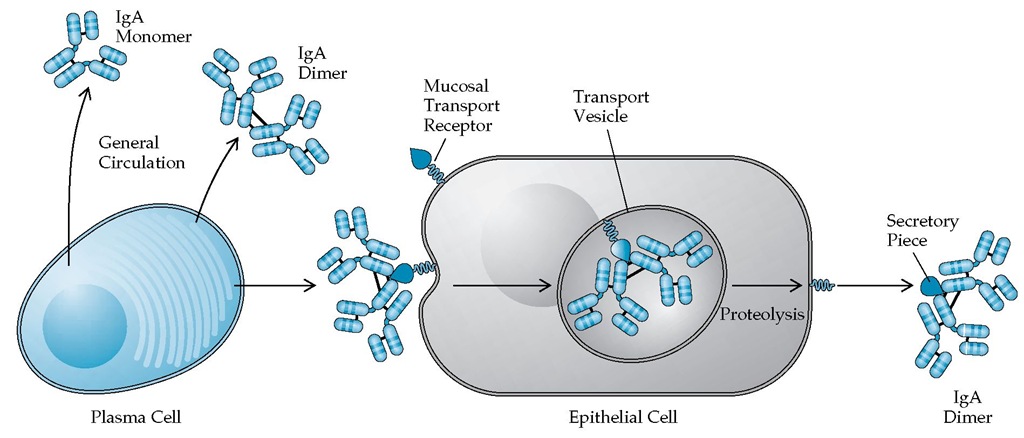
IgA IgA is the predominant immunoglobulin in secretions,where it is usually found as a dimer and is released as such by local plasma cells. Monomeric IgA constitutes 15% of the serum immunoglobulins. In the serum, it has a half-life of 5 to 6 days. There are two subclasses of IgA: IgA1 and IgA2. The IgA dimer combines with the secretory piece, which is a polypeptide chain produced by local epithelial cells. In this form, IgA is quite resistant to proteolytic digestion. Unlike serum IgA, IgA combined with the secretory piece can undergo active transport across the mucosal epithelium by endocytosis [see Figure 2].
IgA is present in saliva, tears, and colostrum. It also occurs in the respiratory and gastrointestinal tracts, in the vagina, and in the prostate. The increased levels of antibodies to dietary antigens that are found in persons with IgA deficiency suggest that the IgA class of immunoglobulin normally limits the absorption of such antigens.
IgA may play an important role in local immunity by neutralizing viruses and by combining with viruses and bacteria, thereby preventing their adherence to mucosal surfaces. Although IgA does not bind to the first component of complement, as IgG and IgM do, it can lead to activation of the alternative complement pathway [see 6:II Innate Immunity]. One of the complement components generated by this pathway, C3b, aids in the opsonization of bacteria, enhancing their uptake and killing by phagocytes.
IgM Most B cells have monomeric IgM on their surface. However, IgM exists primarily as a pentamer and is found mainly in the serum, where it makes up 10% of the im-munoglobulins. In the immune response, IgM is the first im-munoglobulin raised in response to antigen stimulation. Cells that produce IgM or their precursors do not become memory cells, so that a second challenge with antigen produces no more IgM antibody than the first stimulus. Because the IgM response to antigen is short-lived, the presence of specific IgM in the serum may be helpful in establishing the diagnosis of acute infection with a particular pathogen. The fetus makes IgM antibodies to certain microorganisms, which can be helpful in the diagnosis of fetal toxoplasmosis, rubella, or syphilis. Not all fetuses infected by these organisms produce such antibodies, however.
As a pentamer, IgM is highly efficient at fixing complement. Molecule for molecule, IgM is 20 times as effective as IgG in agglutinating bacteria and red blood cells and 1,000 times as active in bactericidal reactions. Isohemagglutinins, such as anti-A and anti-B, are of the IgM class. Waldenstrom macroglobulinemia is a disease characterized by the monoclonal production of IgM.
IgD IgD, which is a monomer, occurs in the serum in trace amounts. It is found in relatively high concentrations in umbilical cord blood. Most of the B cells of umbilical cord blood have IgD on their surface, and most B cells in adults have both IgM and IgD on their surface. Plasma cells that produce IgD have been found in the tonsils and adenoids, although they are very rare in other lymphoid tissues. The function of IgD is unknown.
IgE IgE is present in trace amounts in the serum, constituting only 0.004% of the immunoglobulins. Plasma cells that produce IgE are found in the tonsils and adenoids and on the mu-cosa of the respiratory and GI tracts.
Distinct receptors for IgE are found on the surface of mast cells, B cells, T cells, macrophages, and eosinophils. IgE binds to its receptor on these cells by its Fc portion; heating the antibody destroys its cell-binding ability. Formerly referred to as reagin, IgE plays a primary role in immediate hypersensitivity—name-ly, the immune reaction in hay fever, extrinsic asthma, wheal-and-flare reactions, and anaphylaxis. IgE binds tightly to mast cells and basophils. When these IgE-coated cells interact with specific antigens, termed allergens, they release potent mediators of immediate hypersensitivity, including histamine, slow-reacting substance of anaphylaxis (SRS-A), and an eosinophilic chemotactic factor [see 6:X Allergic Response]. Levels of IgE are higher than normal in persons with atopic dermatitis, as are the levels of IgE antibody specific for allergens to which the individual is susceptible. In patients with allergies, specific IgE antibodies are detected by means of a radioimmunoassay called the ra-dioallergosorbent test (RAST).
The exact function of IgE is unknown. Certainly, the manifestations of immediate hypersensitivity, such as hay fever and extrinsic asthma, do not appear to serve any useful purpose for the person affected or for the species in general. Therefore, the observation that IgE levels are sometimes elevated in persons living in the tropics, and especially in those afflicted with helminthic parasites, was greeted by immunologists as a possible indication that IgE plays a protective role against parasites. The mediators released could affect the parasite either directly or by producing an increase in vascular permeability and the release of eosino-philic chemotactic factor, which may lead to the accumulation of other necessary antibodies (e.g., IgG) and cells to attack the parasite. In this context, it is of interest that eosinophils can mediate IgG-dependent damage to schistosomula (the larval form of the parasite Schistosoma mansoni). In addition, parasite-specific IgE immune complexes can induce a macrophage-mediated cyto-toxic response to schistosomulum organisms.
Antigenic Differences of Immunoglobulins
Immunoglobulins have three types of serologic, or antigenic, determinants: isotypic, allotypic, and idiotypic.
Isotypic determinants Isotypic determinants distinguish between the constant regions of the various classes and subclasses of heavy chains and light chains; they represent the different constant-region genes. For example, there are four IgG heavy-chain isotypes: yi, Y2, Y3, and y4, representing the subclasses IgG1, IgG2, IgG3, and IgG4, respectively. There is only one k light-chain isotype and one X light-chain isotype.
Allotypic determinants Allotypic determinants distinguish between immunoglobulins of a particular isotype; they represent different alleles of immunoglobulin genes and therefore are genetically determined according to mendelian laws in a manner similar to the way that the ABO blood groups are determined. The y heavy chains have more than 20 different allotypic markers, which are collectively termed Gm. In addition, k light chains contain a set of at least three allotypic markers, collectively called Km. There are no known allotypic markers for the m, 8, and e heavy chains or for the X light chain.
Figure 2 Plasma cells secrete IgA molecules into the general circulation as either monomers or dimers. The circulating dimer can combine with a mucosal transport receptor on the surface of an epithelial cell. As the antibody-receptor complex is transported through the cell, the receptor is cleaved. The portion of the receptor that remains attached to the antibody dimer is called the secretory piece. The secretory piece is joined to the constant region of IgA by disulfide bonds. The mucosal transport receptor contains five immunoglobulin-like domains and is anchored in the membrane by a proteolytically labile segment.
Idiotypic determinants An idiotope is defined as a single antigenic determinant on the hypervariable region of an antibody. An idiotype is the antigenic character of the variable region of an antibody. Idiotypic determinants distinguish one im-munoglobulin from another of the same allotype.
Genetic Source of Antibody Diversity
The carboxyl-terminal halves of all k light chains have almost identical amino acid sequences; this portion of the molecule is therefore called the constant, or C, domain. The amino-terminal half has a variable sequence of amino acids and is known as the variable, or V, domain [see Figure 1]. The first 110 amino acids of the amino-terminal portion of the X light chain and the heavy chain are also variable. The remaining 75% of the heavy chain is constant and contains three homologous regions.
Within the variable regions, three areas—referred to as the hy-pervariable, or complementarity-determining, regions—show great variation; these areas correspond to the antigen-binding site of the antibody. X-ray analysis has shown that immunoglob-ulin molecules are built up from compact globular units connected by short segments of more or less linear polypeptide chains [see Figure 1 ]. As expected, the hypervariable regions are located at the interface between immunoglobulin and antigen.
The most intriguing aspect of the genetic control of im-munoglobulin synthesis is the diversity of the product: plasma cells can make antibodies that react with an indefinite number of different antigenic sites. How can DNA code for such a large number of antigens, many of which have only recently (on the evolutionary time scale) come into existence?
VDJ Recombination
In all cells, DNA for the k light chain codes for more than 300 variable (V) regions, five joining (J) regions, and one constant (C) region. The V and J regions are separated from the C region by an intervening stretch of DNA. Thus, in the so-called germline configuration, DNA encodes the information for at least 1,500 different combinations of V and J regions; in other words, at least 1,500 different k light chains are possible.
The emergence of individual plasma cell lines is the result of somatic recombination in the DNA and RNA splicing [see Figure 3]. As the pre-B cell differentiates into a plasma cell, rearrangements and deletions in the DNA bring one of the V genes, chosen at random, adjacent to one of the J genes. This V-J unit and the remaining J regions are separated from the C gene by a short length of DNA. In the next step, the DNA is transcribed to nuclear RNA, and the stage is set for a second event.
This event begins when an enzyme cleaves the nuclear RNA to produce messenger RNA (mRNA). In a process called RNA splicing, the segment that separates the V-J unit from the C region is removed, along with any superfluous J segments. The remaining V-J-C segment is now translated into one of the 1,500 k light-chain proteins. Actually, the number of possible proteins is higher because the joining of any V region to a J region can involve one of a variety of base pairs at the recombination site.
Variability in the heavy chain makes an important contribution to the specificity of an antibody and also results from somatic recombination and RNA splicing. The germline configuration of the DNA carries instructions for several hundred different heavy-chain V genes, six J genes, 10 to 20 diversity (D) genes, and nine C genes (these C genes code for the heavy-chain classes: IgM, IgD, IgG1, IgG2, IgG3, IgG4, IgA1, IgA2, and IgE). DNA deletion, transcription to nuclear RNA, and RNA splicing produce the final V-D-J-C sequence in the mRNA that is translated by ribosomes to a heavy-chain protein. This assembly process produces more than 18,000 possible varieties of heavy-chain proteins (antibody specificity does not vary with class, so the nine C genes do not enter into the calculation).
The combination of more than 1,500 light-chain varieties with the 18,000 heavy-chain varieties yields more than 27 million different kinds of antibodies with different antigen-binding sites. In addition, somatic hypermutation occurs, particularly during affinity maturation, and the rate of somatic hyper-mutation is relatively high (one base pair per 1,000 cell divisions). Therefore, the potential number of specific antibodies in a single person is indefinite.
Although some of the mechanisms of V-D-J rearrangement are unique to B cells (and T cells, as synthesis of TCRs occurs by a similar mechanism [see T Cell Receptors, below]), the general mechanisms of DNA repair are also engaged.2 Two genes involved in V-D-J rearrangement in B cells are the recombination-activating genes rag1 and rag2.35 Disruption of the rag1 or rag2 gene causes a block in B cell development before the transition from pro-B cell to pre-B cell, as found in patients with severe combined immunodeficiency syndrome or Omenn syndrome.6 Disruption of the surface IgM gene, the J region of the heavy chain, or the J region of the k light chain leads to a similar block in B cell development.
Allelic Exclusion
In an individual B cell, only one of the chromosomes undergoes complete V-D-J recombination leading to expression of heavy and light chains. A mechanism exists that prevents the other chromosome from being rearranged and therefore expressed in the same cell. This is called allelic exclusion. It prevents a B cell from expressing two entirely different immu-noglobulins or BCRs. A similar mechanism operates during synthesis of TCRs in T cells [see T Cell Receptors, below].
Primary and Secondary Antibody Responses
When antigen is first introduced into the body, a primary response occurs that is characterized by a lag phase that lasts several days, during which no antibody is detected. Increasing amounts of IgM antibody appear in the serum, usually reaching a peak level after 7 days. After 6 to 7 days, IgG antibody is also detected. The IgM titer begins to wane before the maximal IgG titer is reached, about 10 to 14 days after the antigen is introduced. Antibody titers then decrease, and very little antibody is detected 4 to 5 weeks after a single dose of antigen.
If the antigen is encountered a second time, a secondary response (also called an anamnestic or booster response) occurs because of the existence of memory B cells. Both IgM and IgG titers rise exponentially, without the lag phase seen in the primary response. Whereas the peak IgM level during the secondary response may be the same as, or slightly higher than, the peak IgM level during the primary response, the IgG peak level during the secondary response is much greater and lasts longer than the peak level during the primary response. This variation in response is an apt illustration of immunologic memory and is caused by a proliferation of antigen-specific B cells and helper T cells during the primary response. The characteristics of the primary and secondary responses explain the need for booster injections in immunization programs.
Figure 3 Variable-region genes are constructed from gene segments. Light-chain variable genes are constructed from two segments (center panel). A variable (V) and a joining (J) gene segment in the genomic DNA are joined to form a complete light-chain variable-gene-region gene. The constant region is encoded in a separate exon and is joined to the variable-region gene by RNA splicing of the light-chain message to remove the L to V and the J to C introns. Immunoglobulin chains are extracellular proteins, and the V gene segment is preceded by an exon encoding a leader peptide (L), which directs the protein into the cell’s secretory pathways and is then cleaved. Heavy-chain variable regions are constructed from three gene segments (right panel). First the diversity (D) and J gene segments join, then the V gene segment joins to the combined DJ sequence, all at the genomic DNA level. The heavy-chain constant-region sequences are encoded in several exons: note the separate exon encoding the hinge domain (purple). The constant-region exons together with the L sequence are spliced to the variable-domain sequence during processing of the heavy-chain gene RNA transcript. Posttranslational alterations remove the L sequence and attach carbohydrate moieties.