1. Introduction
Phosphorylation is one of the most prevalent and important intracellular protein modifications. It is estimated that at any given time approximately 30% of all proteins are phosphorylated (Hunter, 1998). The fast reversibility of protein phosphorylation makes it very attractive for regulating cellular processes as diverse as metabolism, signaling, and cell proliferation. Numerous amino acid residues can be phosphorylated including aspartic acid, cysteine, and histidine. However, major research efforts so far have been focused on serine, threonine, and tyrosine phosphorylation because these amino acid residues are the main target for phosphorylation in eukaryotes. In addition, they are chemically more stable and thus considerably easier to analyze (Yan etal., 1998). Phosphorylation analysis is composed of several steps: (1) the detection of a known phosphorylation site, (2) residue-resolved localization of (novel) phosphorylation sites, (3) monitoring changes in phosphorylation (relative quantitation), and (4) determining the degree of phosphorylation (absolute quantitation of phosphosylation stoichiometry).
Common strategies for the analysis of protein phosphorylation utilize radioactive phosphorus isotopes, phosphospecific or phosphorylation site specific antibodies, and/or mass spectrometry (MS). Each method has its advantages and disadvantages for phosphorylation analysis, which will be summarized in the following section of this article.
2. Radioactive labeling
A well-established method for the analysis of protein phosphorylation is the incorporation of the radioactive phosphorus isotopes 32P or 33P. In order to detect a particular phosphorylation site, this method is combined with phosphopeptide mapping by two-dimensional thin layer chromatography (2D-TLC) or liquid chromatography (LC) followed by radioactivity measurements to identify the spots/fractions of interest (Yan etal., 1998). However, this approach requires that a detailed analysis of the different phosphopeptides is carried out in order to unambiguously correlate a spot/fraction with a particular phosphorylation site. Since the detection of radioactivity is quantitative this method is suitable for relative quantitation, but not necessarily for absolute quantitation as the initial amount of protein is not always known.
To utilize radioactive labeling for residue-resolved localization of phosphoryla-tion sites, Edman degradation-based approaches have proven to be useful. After isolating and purifying the phosphopeptides by, for example, 2D-TLC or LC, the identity of the peptide is determined by MS. This is followed by Edman degradation where the peptide is sequentially hydrolyzed and the release of radioactivity during one particular cycle from the immobilized peptide can be correlated with the primary sequence as determined by MS prior to the Edman degradation. The sequence of events described above identifies the site of phosphorylation (Campbell and Morrice, 2002).
True “de novo” sequencing of phosphopeptides by Edman degradation is problematic as the Phenylthiohydantoin (PTH) derivative of phosphotyrosine is barely soluble (Aebersold et al., 1991) and phosphoesters of threonine and serine undergo j-elimination under the conditions used for Edman sequencing. This side reaction gives rise to numerous undefined products such that phosphoserine or phosphothreonine residues are often only assigned with ambiguity (Campbell et al., 1986; Dedner etal., 1988).
Radioactive labeling is very sensitive and well suited for in vitro phosphorylation assays. However, in vivo incorporation of the radioactive isotopes is not possible in the case of tissue samples or is very inefficient in the case of cell culture due to the presence of endogenous unlabeled adenosine triphosphate (ATP). Hence, to achieve a degree of in vivo phosphorylation that is sufficient for sensitive detection, very large amounts of radioactively labeled ATP are required such that other pleiotropic effects cannot be excluded.
3. Phosphospecific antibodies
Three different types of antibodies are used for protein phosphorylation analysis:
1. Anti-phosphoamino acid antibodies: Anti-phosphotyrosine antibodies were invaluable for the elucidation of phosphotyrosine-based mitogen and cytokine signaling. They can be used for profiling the global tyrosine phosphorylation state of a particular sample after 1D or 2D gel analysis (see Article 22, Two-dimensional gel electrophoresis, Volume 5; Figure 1b), and for the enrichment of tyrosine-phosphorylated proteins by immunoprecipitation (Pandey et al., 2000). Although antibodies are mainly used for proteins, a recent study showed that these antibodies are also applicable to the enrichment of tyrosine-phosphorylated peptides (Rush etal., 2005). The development of good anti-phosphoserine and anti-phosphothreonine antibodies has been less successful so far (Gronborg etal., 2002).
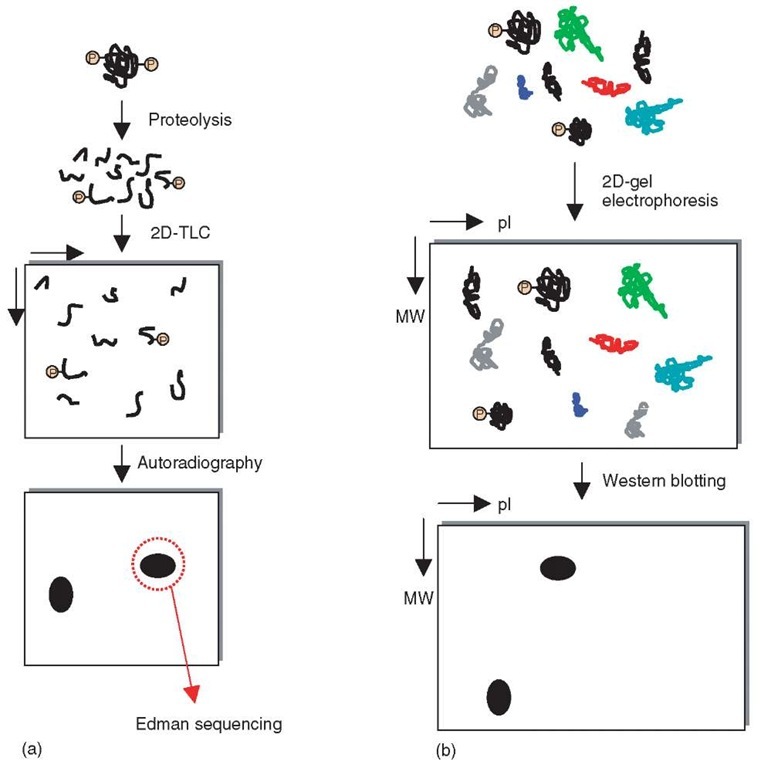
Figure 1 (a) A schematic portraying the radioactivity-based analysis of phosphorylated peptides involving proteolysis, two-dimensional thin layer chromatography (2D-TLC), and autoradiography to visualize the radioactively labeled peptides prior to Edman sequencing to determine the amino acid sequence. (b) A schematic for the identification of, for example, tyrosine-phosphorylated peptides using western blotting with anti-phosphotyrosine antibodies subsequent to two-dimensional gel electrophoresis (2D-GE). 2D-GE separates proteins in the first dimension on the basis of the isoelectric point (pI) and in the second dimension, on the basis of molecular weight
2. Antibodies against phosphorylation consensus motifs: These antibodies are raised against oriented peptide libraries mimicking particular phosphorylation consensus motifs (available from e.g., Cell Signaling Technology). They are a suitable compromise for serine and threonine phosphorylation motifs and can be used in a fairly broad context as well as for specific questions (Zhang et al., 2002a).
3. Phosphorylation site-specific antibodies: As antibodies can be the most sensitive, specific, and fastest way to detect a particular protein phosphorylation in complex protein mixtures, such as whole cell lysates, the use of these types of antibodies is becoming more widespread. However, localization of the phosphorylation site by other means such as MS, Edman degradation with radioactive labeling is a requirement because the unbiased localization of novel phosphorylation sites using site specific antibodies is not possible.
Another advantage of antibodies is the semiquantitative nature of the Western blotting such that relative (semi-)quantitation is possible. However, absolute quantitation is impossible if one only uses phosphospecific antibodies. Other limitations of the use of antibodies stem from the fact that it is not always possible to raise antibodies against all phosphorylation sites and that specificities can be questionable in cases where multiple phosphorylation sites are found in close proximity.
4. Mass spectrometry
Mass spectrometry is very commonly used for the analysis of protein phosphory-lation especially due to its improved sensitivity and speed as compared to many traditional biochemical methods such as Edman degradation. Another major advantage of MS is its versatility – that is, it can be used for any kind of phosphorylation analysis. If the identity of a protein and its phosphorylation sites are known, then directed mass spectrometric experiments can be performed in order to determine whether a particular site is phosphorylated or not as the mass-to-charge ratio of the expected proteolytic phosphopeptide can be calculated.
1. Relative quantitation of protein phosphorylation can be performed using stable isotope labeling approaches using metabolic, enzymatic, or chemical means to incorporate the label (Ibarrola etal., 2003; Oda etal., 1999). Alternatively, stable isotope-free methods can be used to monitor changes in the degree of phosphorylation after normalizing the raw data to account for run-to-run variations and variations in the starting amount of material (Ruse et al., 2002; Steen et al., 2005).
2. Absolute quantitation of the phosphorylation stoichiometry can be achieved by using either isotopically labeled internal standards (Stemmann etal., 2001), isotope labeling in combination with phosphatase treatment (Zhang etal., 2002b), or a completely stable isotope-free methods (Steen et al., 2005).
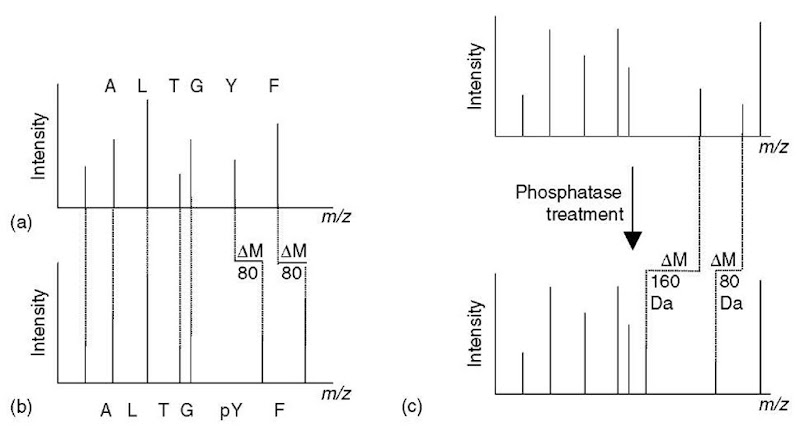
The true power of MS is its ability to sequence peptides in a second to subsecond timescale and to unambiguously localize phosphorylation sites down to the residue level in an unbiased manner (Figure 3a and b; Article 4, Interpreting tandem mass spectra of peptides, Volume 5). Different strategies have been devised to identify and analyze the phosphorylated peptides within a complex mixture such as proteolytic protein digests.
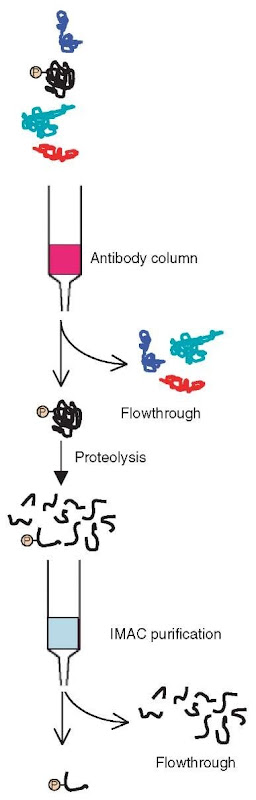
The most successful strategies for mass spectrometric analysis of protein phosphorylation is to utilize either selective enrichment of phosphorylated species utilizing, for example, generic antibodies (see above) or immobilized metal affinity chromatography (IMAC; see below) approaches (Figure 2) (Posewitz and Tempst, 1999) and/or utilize the characteristic fragmentations of phosphopeptides upon collisionally induced dissociation (CID) for so-called (constant) neutral loss and/or precursor ion experiments (Covey et al., 1991; Huddleston etal., 1993; see also Article 18, Techniques for ion dissociation (fragmentation) and scanning in MS/MS, Volume 5).
Figure 2 A general strategy using antibodies and immobilized metal affinity chromatography (IMAC) to enrich for phosphoproteins and subsequently phosphopeptides. Each of the enrichment steps can also be used separately
In general, it is always advisable to reduce the complexity of the sample as much as possible by enriching the protein(s) of interest or even the phosphorylated form(s) of the protein(s) of interest using, for example, antibodies. However, it should be noted that quantitation of the phosphorylation stoichiometry is not possible if only the phosphorylated species (protein or peptide) is enriched. Following the enrichment of the protein(s) of interest, the sample is digested generating a complex mixture of peptides some of them being modified, that is, phosphorylated, but the vast majority being unmodified. The analytical challenge is to maximize the time spent on the analysis of the phosphopeptides and to minimize the time spent on the unmodified peptides. However, a bias against substoichiometric species of low abundance has to be expected when simple data dependent acquisition (DDA) routines are used for the acquisition of data since the peptides are selected for sequencing simply on the basis of the signal intensities of the peptide in the survey scan. This is less of a problem for samples of fairly low complexity, for example, single protein digests, as long as the number of peptides eluting off the LC column does not exceed the number of peptides that can be analyzed with these DDA routines. Thus, although a simple DDA experiment with a standard LC setup is valid as a first screen/first pass experiment, one must exercise caution. If phosphopeptides of very low abundance are expected or the sample is highly complex, such that at any given time during an LC/MS experiment more peptides elute than can be analyzed using DDA routines, numerous phosphopeptides might not be sequenced. Then, either enrichment protocols aimed at enriching the phosphopeptides or selective detection methods are advisable to efficiently utilize the valuable measuring time for analysis of the species of interest.
4.1. Selective detection
The phosphopeptide-specific mass spectrometric detection is based on characteristic fragmentations of phosphopeptides when fragmenting them in tandem mass spectrometers. For instance, in the negative ion mode (which is generally not suitable for peptide sequencing), a highly characteristic fragment ion at m/z -79 corresponding to PO3- is observable. In contrast, in the positive ion mode, a characteristic loss of H3PO4 (98 Da) is often prevalent for phosphoserine-and threonine-containing peptides, but is not commonly observed for tyrosine-phosphorylated peptides. The novel linear ion trap instruments (QTRAP or LTQ) are especially useful in utilizing these phosphopeptide-specific features on an LC-compatible timescale (Beausoleil et al., 2004; Le Blanc et al., 2003).
4.2. Selective enrichment
The most commonly used enrichment method is based on IMAC, which selectively binds phosphopeptides. Commercially available and in-house-made IMAC materials are commonly used and have all shown promising results. However, because of the mode of binding, it is expected that a certain number of acidic peptides, that is, aspartic acid and glutamic acid-rich peptides will be enriched as well. This problem of nonspecificity is more prevalent with highly complex samples (e.g., whole cell lysates) such that esterification of the carboxyl groups is recommended in those cases (Brill et al., 2004; Ficarro et al., 2002).
Other enrichment methods that are easily implemented are based on strong cation exchange (SCX) (Beausoleil et al., 2004) or strong anion exchange materials (SAX) (Nuhse et al., 2003). Ion exchange chromatography approaches utilize the low/high affinity of phosphopeptides to the respective ion exchange material. Both studies show impressive results using highly complex peptide mixtures as starting material. However, the binding/nonbinding of both the SCX and SAX approaches rely on the net charge of the peptide, that is, the amino acid composition. Thus, they are biased against certain subsets of peptides, for example, histidine-containing phosphopeptides in the case of SCX-based strategies (see Article 13, Multidimensional liquid chromatography tandem mass spectrometry for biological discovery, Volume 5).
If matrix assisted laser desorption/ionization (MALDI) is employed for the phosphopeptide analysis, then “hot” (dissociation-inducing) matrices such as a-cyano-4-hydroxycinnamic acid should be avoided as they lead to the loss of the phospho moiety from serine and threonine-phosphorylated peptides, reducing the signal intensity of species of interest, that is, the phosphopeptides (see Article 14, Sample preparation for MALDI and electrospray, Volume 5). Thus, the use of “cold” matrices such as dihydroxybenzoic acid are recommended. Also, the addition of nitric or phosphoric acid has shown to increase the ionization/detection efficiencies of phosphopeptides (Stensballe and Jensen, 2004). If a MALDI mass spectrometer without MS/MS capability is used for the MALDI analysis, phosphopeptides can be selectively identified in peptide mixtures by performing the MALDI MS analysis before and after phosphatase treatment of the sample. The phosphorylated species decreases in weight by 80 Da or multiples thereof upon enzymatic dephosphorylation (Figure 3c). However, for residue-resolved localization of the phosphorylation sites, additional MS/MS experiments on the species of interest are required.
5. Other methods
Instead of using generic antibodies against phosphoamino acid to profile the phosphorylation state of samples after 1D or 2D gel analysis, it is also possible to use a phosphospecific fluorescence dye called Pro-Q Diamond (Molecular Probes) (see Article 27, Detecting protein posttranslational modifications using small molecule probes and multiwavelength imaging devices, Volume 5). This reagent provides detection limits in the subpicomole range and is compatible with subsequent in-gel digestion and MS analysis protocols. Hence, more detailed analysis of the gel spots of interest is possible. Furthermore, the binding of the fluorescence probe can be used for quantitation up to 3 orders of magnitude (Steinberg et al., 2003).
6. Conclusion
The methods described above are only a small selection of a vast array of different strategies used for the analysis of protein phosphorylation. The aim of this tutorial is to give a brief synopsis of the most broadly applicable and promising detection and analytical methods, as well as their strengths and limitations. Experimental details should be explored in the references given. All of the methods have their particular advantages, such that the choice of method should be based on the question asked. An additional implication is that none of the methods is likely to provide a complete picture of the protein phosphorylation of a particular protein. For more comprehensive analysis, several methods should be combined as demonstrated by Vihinen and Saarinen (2000), and Chen etal. (2002) who combined five and more different strategies.
Figure 3 A schematic portraying how to localize phosphorylation sites using mass spectrometric phosphopeptide sequencing. Sequence-revealing peptide fragment ions derived from the phosphopeptide (b), that carry the phospho moiety and thereby localize the site of phosphorylation, are 80 Da heavier than the corresponding fragment ions in the product-ion spectrum of the unmodified cognate (a). (c) Identification of phosphopeptides within a peptide mixture based on the mass shift induced by phosphatase treatment of the peptides.