1. That
The difference between pro- and eukaryotes suddenly appeared smaller when in 1974 Mescher and Strominger demonstrated that prokaryotes also could glycosylate their polypeptides. Initial work was on Archeae (like Halobacterium halobium) but later studies demonstrated that less exotic Eubacteria behaved similarly, and the Slayer glycoproteins were for years paradigmatic. More recently, it has become clear that also many common pathogens such as Campylobacter jejuni, Neisseria meningitidis , Mycobacterium tuberculosis, and Pseudomonas aeruginosa glycosylate polypeptides, often modifying outer membrane proteins (OMPs) or well-recognized virulence factors such as pili or flagella (Messner and Schaffner, 2003; Power and Reeves, 2003; Schmidt etal., 2003; Szymanski etal., 2003). Thus, the ability to express glycoproteins is widely distributed over the prokaryote kingdom.
2. What
As in eukaryotes, prokaryote glycans can occur O-linked to serine/threonine/tyrosine or N-linked through asparagine (see Article 64, Structure/function of N -glycans, Volume 6, Article 65, Structure/function of O -glycans, Volume 6, and Article 76, Analysis of N- and O-linked glycans of glycoproteins, Volume 6). The eukary-otic consensus motif for N-glycosylation (Ser/Thr-X-Asn) has also been found in prokaryotes. While in eukaryotes, the (N-linked) glycan chains can be grouped into complexes, high mannose and hybrid, diversity in prokaryotes precludes such grouping. Their glycan substitutions vary between simple monosaccharides (Man, GlcNAc, GalNac) and complex oligosaccharides (n > 15) that include residues not encountered in man (like pseudaminic acid, an analog of sialic acid); these glycans may even consist of repeating units. Finally, to come full circle, high man-nose glycans (8/9 mannoses), similar to those found in eukaryotes are expressed by the intracellular parasite Chlamydia trachomatis. Thus, prokaryotes protein glycans show a bewildering diversity and are sometimes completely eukaryotic-like.
3. How
The machinery required for glycosylating bacterial proteins has been elucidated recently in various species, and is best exemplified by C. jejuni, in which separate pathways for O- and N-glycosylation have been identified (Szymanski et al., 2003). These pathways are also physically separated on the chromosome, each organized in a glycosylation island. The O-glycosylation island (locus) consists of about 50 genes (between Cj1293 and Cj1337 in the genome strain 11168) and is adjacent to the flagellin structural genes flaA and flaB. Surprisingly, for this size of locus, the glycan chains of flagellin consist of a monosaccharide only (pseudaminic acid and derivatives). However, the degree of glycosylation is unsurpassed for a bacterial protein: 19 Ser/Thr residues carry this monosaccharide (=10% of the MW of native flagellin). For several genes in this locus, a likely role in the biosynthesis of pseudaminic acid could be assigned. For instance, Cj1293 codes for a functional UDP-GlcNAc dehydratase, Cj1313 and Cj1321 are acetyltransferases, and Cj1331 (called ptmB) is a CMP-NANA synthetase homolog, hence is likely to be involved in synthesis of the pseudaminic acid-CMP, the hypothesized glycosyl donor of this residue. Strikingly however, the glycosyltransferase itself has not been identified yet.
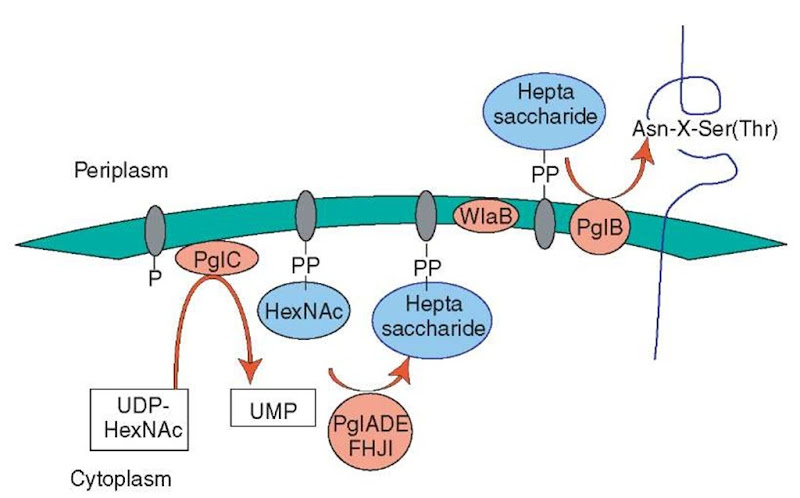
The N-glycosylation locus (also called pgl, for protein glycosylation) is much smaller (Cj1119c-1130c) but puts on a much more complex chain, namely, the heptasaccharide GalNAc-a1,4-GalNAc-a1,4-[Glc^ 1,3]GalNAc-a1,4-GalNAc-a1,4-GalNAc-a1,3-bacillosamine (bacillosamine=2,4-diacetamido-2,4,6-trideoxyglucose). This machinery is also called the general glycosylation pathway, as it modifies >30 polypeptides that were identified after affinity chromatogra-phy with the GalNAc-specific lectin soybean agglutinin and SDS-PAGE. Mechanistic insight on how these glycans are synthesized was obtained though the identification of the Cj1126c (also called pglB) as an oligosaccharidyltransferase, highly homologous to its eukaryotic (yeast) counterpart STT3. STT3 transfers completed lipid-bound oligosaccharides to the Asn, as present in the consensus motif Asn-X-Ser/Thr. On the basis of analogy with eukaryotic N-glycan synthesis, the following scenario for C. jejuni N-glycan synthesis seems plausible (Figure 1): pglC (Cj1124c) first attaches an acetylhexosamine (starting with a UDP-acetylhexosamine donor) to a membrane-inserted polyprenyl lipid carrier; the subsequent actions of the pglF and other gene products of this locus, which contains at least five glycosyltransferases, form the complete heptasaccharide. Then, in analogy with eukaryotes, the lipid-bound heptasaccharide flip-flops from cytoplasmic to the periplasmic side of the membrane (by Cj1130c, WlaB, an ABC-transporter). The final step takes place when PglB transfers the heptasaccharide to Asn.
While in C. jejuni separate pathways exist for protein- and lipid glycosylation, in P. aeruginosa these routes intersect and the same trisaccharide is present on pilin and in LPS. Transfer of the trisaccharide from a lipid carrier toward pilin by a rather nonspecific oligosaccharidyltransferase (pilO) seems likely; hence, this route has similarities to the N-glycosylation system of C. jejuni.
Thus, the prokaryotic glycosylation machinery has been elucidated in quite substantial detail, in particular through whole-genome sequencing but not always functional data (enzyme activities) are available. Clearly, the N-glycosylation pathway, formerly thought to be present in eukaryotes only, is present in Prokaryotes.
Figure 1 Model for the biosynthesis of N-linked glycoproteins in Campylobacter jejuni (adapted from Szymanski CM, Logan SM, Linton D and Wren B (2003) Campylobacter jejuni-a tale of two glycosylation systems. Trends in Microbiology, 11, 233-238). Nucleotide-activated sugars are assembled on a lipid carrier, which results in the formation of a specific heptasaccharide. Subsequently, the glycan moiety is flipped across the inner membrane by WlaB (Cj1130c) and transferred to the appropriate asparagine residue by PglB. P: lipid-carrier phosphate; PP: lipid-carrier pyrophosphate; UDP: uridine diphosphate; UMP: uridine monophosphate; HexNAc: acetylhexosamine
4. Why
Recent data suggest that bacterial protein glycosylation has, apart from a stabilizing or structural role, an important role in the adaptation of prokaryotes to their environment, like host-pathogen interaction. Two examples are given below.
4.1. The role of pilin glycosylation in gonococcal pathogenesis
Neisseria gonorrhoeae is the causative agent of gonorrhoea, a disease that can cause major complications upon be dissemination. PilE, the major structural pilin protein, is O-glycosylated with Gala 1.3GlcNAc, with pgtA coding for the galactosyltransferase (Banerjee etal., 2002). In pgtA, a poly G-tract can be present, a signature for phase variation through DNA slippage (see Article 62, The neisserial genomes: what they reveal about the diversity and behavior of these species, Volume 4) (slipped-strand mispairing). When, for instance, a G14 tract is present in the parent bacterial cell, an intact GalT is formed and a disaccharide glycan is present. Upon cell division, owing to DNA slippage, phase-variant daughter cells arise with a G15 tract, which causes downstream frame-shifting and a concomitant lack of a functional GalT and of galactosylated pili. Strikingly, strains that cause noncomplicated gonorrhea (i.e., confined to genitourinary epithelium) lack G-tracts (pili subunits are always modified), while disseminating strains display phase variation. A plausible mechanism is that the gonococci are recognized by an epithelial lectin and that for invasiveness prior detachment through phase variation is essential. Alternatively, the disaccharide might be the target of bactericidal serum antibodies, and hence phase variation would serve immune evasion.
4.2. The role of Pseudomonas syringae flagellin glycosylation in host specificity
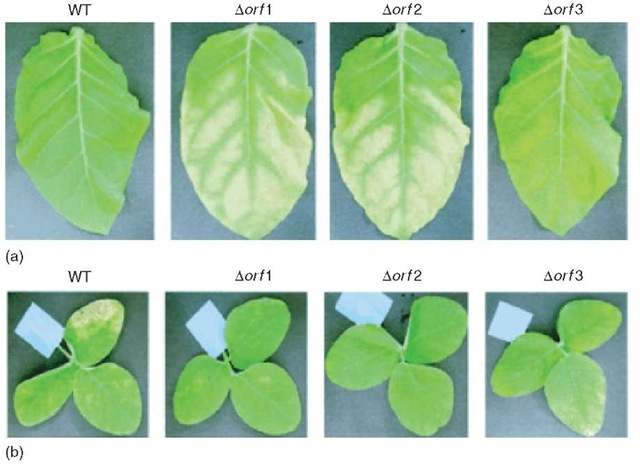
Pseudomonas syringae is a plant pathogen of tobacco, soybean, tomato and others. Within P. syringae, pathovars (pv.) exist with strict host specificity (Takeuchi etal., 2003): while P. syringae pv. tabaci can infect its host the tobacco plant (compatibility), it is not compatible with nonhost plants such as soybean; vice versa, P. syringae pv. glycinea is compatible with soybean but not with tobacco. When a pathovar encounters a noncompatible host, a host defense (hypersensitivity, HR) reaction occurs, involving host cell apoptosis. Glycosylated pili are responsible for this HR reaction. In P. syringae pv. glycinea, a small glycosylation island (three orfs of which two are glycosyltransferases) was detected between two flagellin structural genes fliC and fliL. Disruption of the glycosyltransferases led to the production of non-/less-glycosylated pili and compatibility with tobacco, that is, this bacterium did no longer elicit the HR (Figure 2) reaction and could cause symptoms in this normally nonhost. Conversely, the mutants did not infect their original soybean host any more. These data prove that specific glycosylation and recognition is responsible for host specificity.
5. Now what?
By necessity, the study of prokaryote glycosylation started as “stamp collection”, that is, collecting structures of sometimes complex prokaryote glycan residues or chains. Now, the field is rapidly moving ahead into understanding the functional significance of glycosylation. The driving force is the explosion of -omics, including genomics, proteomics, and glycomics (Drickamer and Dell, 2002; see also Article 74, Glycoproteomics, Volume 6 and Article 75, Mass spectrometry, Volume 6). Protein spots present in 2D gels can be picked and, owing to the revolution in mass spectrometry (high throughput, enhanced sensitivity), be identified almost momentarily, compared to whole genomes, and assigned a function through increasingly sophisticated bioinformatics. In parallel, the field benefits from developments in carbohydrate synthesis (oligosaccharide libraries and glyco-chips). From the picture that is now emerging, it becomes clear that as in eukaryotes, prokaryote glycans can serve as information carriers, fine-tuning the interplay between prokaryotes and their environment, including human and nonhuman hosts.
Figure 2 The role of pilin glycosylation on compatibility of Pseudomonas syringae pv. glycinea with tobacco (a) and soy (b) (Reproduced from Takeuchi K, Taguchi F, Inagaki Y, Toyoda K, Shraishi T and Ichose Y (2003) Flagellin glycosylation in Pseudomonas syringae pv. glycinea and its role in host specificity. Journal of Bacteriology, 185, 6658-6665 by permission of American Society for Microbiology). Wild-type Pseudomonas syringae pv. glycinea is not compatible with (i.e., cannot infect) its nonhost tobacco but is so with its host soy, where it causes symptoms (whitish discoloration). After inactivation of glycosyltransferases required for pilin glycosylation (Aorf1 or Aorf2), mutants are compatible with tobacco but now compatibility with the original host soy is lost