KEY CONCEPTS:
Upon completion of this topic, it is expected that the reader will understand these following concepts:
• Maintenance of cardiac rate, rhythm, and pumping ability as support for the brain
• Autonomic nervous system control
• Mechanisms of action of drugs affecting the heart, lungs, and kidneys
CASE STUDY:
Mrs. Fein called 9-1-1 because she felt faint and very fatigued. One Paramedic interviewed Mrs. Fein while her partner scanned the medication bottles. "Do you take all of these medications?" one Paramedic asked. Mrs. Fein answered, "Oh yes. I always do what my doctors tell me to do. I am so glad that I have so many fine doctors to take care of me."
There were multiple antihypertensives, antidysrhythmics, and diuretics. At least four different pharmacies had filled the prescriptions. "I see that you go to several pharmacies to have your prescriptions filled," said the Paramedic. Mrs. Fein replied, "Well, each of my daughters likes a different pharmacy and they often pick up my prescriptions for me."
OVERVIEW
The brain, as the source of one’s being and the seat of one’s consciousness, is the most important organ in the body. Perhaps the two most important support systems for the brain in the body are the heart and lungs. These two organs, through constant adjustment and readjustment, ensure that the brain gets sufficient oxygen and perfusion of glucose-rich blood in order to function. Any disequilibrium between the heart and lungs results in cerebral hypoxia, hypoglycemia, or hypoperfusion. Persistent hypoxia, hypoglycemia, or hypoperfusion can lead to an alteration in mental status, loss of consciousness, and eventually death.
Paramedics are frequently called to treat a patient with loss of consciousness, shortness of breath, or cardiac-related problems. The importance of these two interconnected organ systems to the patient’s health cannot be understated. Paramedics must have an intimate understanding of the heart and lung systems and the treatments which they can provide to support them.
The Nervous System
The brain controls these two vital organ systems through the autonomic nervous system. Therefore, cardiopulmonary pharmacology is focused on affecting the autonomic nervous system. To understand the effects of cardiopulmonary pharmacology, the Paramedic must have an expansive knowledge of the autonomic nervous system.
In about 200 A.D., Galen, the father of medicine, identified something "non-tendon" in the muscle. He had identified a nerve.1 Later, anatomists would note that stimulation of these nerves caused muscle movement and they sought to discover what other functions nerves provided.
In the mid-1900s, Dr. William Cullen advanced the idea that the nervous system was responsible for maintaining the physiological balance of all organs within the body. He was correct. The nervous system is responsible for the regulation of body functions. Through an intricate system of wire-like fibers, called neurons, which are present throughout the body, messages are sent which stimulate the cells within the organs to respond.
The Central Nervous System
The central nervous system, which consists of the brain and the spinal cord, is analogous to the command and control center of an army. Information, or intelligence, from the outside world flows through the spinal cord to the brain to be processed. In many instances, the brain sends a command (a signal) to the organs to respond in a certain manner, via the peripheral nervous system.
The Peripheral Nervous System
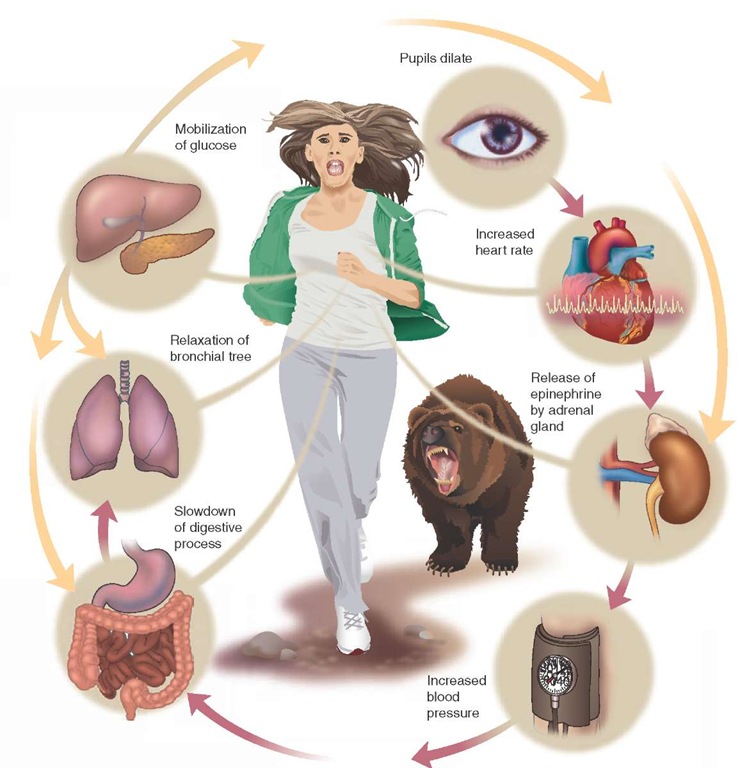
The peripheral nervous system consists of the 12 cranial nerves and the 31 spinal nerves that extend from the brain and spinal cord to the organs of the body.2 Similar to a two-lane highway, information flows to and from the brain along the peripheral nervous system. The afferent division is the portion of the peripheral nervous system that is stimulated by the environment (e.g., by heat or by touch) and sends a signal to the central nervous system. The central nervous system, in turn, interprets the data and sends a signal via efferent nerve fibers to the body to react. In some cases, the act is voluntary (e.g., to pat a dog’s head). In other cases, the act is involuntary (e.g., a quicker heartbeat when faced with the threat of a menacing bear). This involuntary control is a function of the autonomic nervous system.
Autonomic Nervous System
The autonomic nervous system can be thought of as the body’s autopilot.3 Essential, life-preserving functions, such as digestion, are maintained by the autonomic nervous system. The autonomic nervous system is further divided into two divisions: the sympathetic division and the parasympathetic division. These two divisions of the autonomic nervous system compete, to some degree, with one another in order to maintain equilibrium while adjusting to external and internal stress.
The sympathetic division of the autonomic nervous system, whose nerve fibers originate in the thoracic or lumbar area of the spinal cord, serves to accelerate the body’s organs. Referred to as the "fight or flight" response, the sympathetic nervous system increases heart rate, dilates the bronchioles to allow more air movement, and constricts blood vessels, causing the shunting of blood to the vital core organs.4-7 Because of its "crisis" orientation, the sympathetic nervous system tends to create an "all or nothing" response, meaning it simultaneously stimulates all of its target organs.
The parasympathetic division of the autonomic nervous system, whose nerve fibers originate and extend from the cervical or sacral area of the spinal cord, is responsible for the more vegetative functions. Referred to as the "feed or breed" portion of the nervous system, the parasympathetic nervous system increases gastric motility as well as stimulates erections in men.
The vagus nerve (from the Latin word meaning "wandering") is the major parasympathetic nerve. The vagus nerve originates in the medulla, exits the skull at the base of the brain, travels down the neck (proximal to the larynx), branches into the heart and lungs, innervates the stomach, passes through the digestive tract, and ends in the anus.
Most organs have dual innervations, both sympathetic and parasympathetic. However, the parasympathetic nervous system usually dominates. For example, the upper portion of the heart, the atrium, has both sympathetic and parasympa-thetic nerve fibers. Yet the parasympathetic nerve, the vagus nerve, dominates, creating a "vagal tone."
Certain select organs only have sympathetic innervation. For example, the adrenal medulla (which excretes the hormone adrenaline), the kidney, and the lower portion of the heart (ventricles) are innervated by sympathetic nerve fibers only.
Neurotransmitters
The autonomic nervous system transmits its signal to the target organ (the effector organ), causing the organ to act in response to the signal. The transmission of the signal from the nerve to the organ is by means of a messenger called a neurotransmitter. There are many neurotransmitters in the central nervous system.
The chief neurotransmitter for the sympathetic nervous system is norepinephrine, which is chemically similar to the hormone adrenaline. Because of its utilization of an adrenaline-like chemical, these nerves are also called adrenergic nerves.
The chief neurotransmitter for the parasympathetic nervous system is acetylcholine. Because of its use of acetylcholine, these nerves are also called cholinergic nerves. These terms—"adrenergic" and "cholinergic"—are important to understanding some descriptions of drug effects.
For each neurotransmitter, there is a corresponding neuroreceptor that receives the neurotransmitter, chemically connecting with it in a key and lock-like fashion. The linkage of neurotransmitter to cell receptor can cause a cell to change the conductivity of an ion channel in the cell wall, thus making it more or less responsive to a stimulus.
For example, norepinephrine can cause the cell to open its potassium channels, which in turn causes a cascade of events, called depolarization.810 This collectively causes the heart to contract quicker and stronger.
Alternatively, the neurotransmitter can stimulate a protein to perform a certain intracellular function. Serotonin, a neurotransmitter found primarily in the gastrointestinal tract, is also present in platelets and within the brain. Serotonin, released by damaged platelets, causes arterial and venous constriction; this is thought to be one of the causes of migraine headaches.11-14 Serotonin within the brain is primarily located in the hypothalamus, where it affects sleep, temperature, pain perception, and mood. Its impact on mood is an important feature which many anti-depressant medications depend on for their effectiveness (see MAO inhibitors and selective serotonin re-uptake inhibitors).
Neurotransmission
The process of neurotransmission is a cycle (Figure 30-1). Understanding this is the key to understanding the drugs which can affect the autonomic nervous system. The phases in the cycle are preparation for action, feedback, and preparation for another action. The speed or strength of a cycle can be increased or decreased by a drug’s influence during that cycle.
To review, the nerve ending makes and stores neurotransmitter in pockets called "vesicles" in the terminal end of the neuron. With stimulation, the neurotransmitter is released into the space between the nerve and the target cell, called the synapse. In the synapse, the neurotransmitter floats over to the cell and attaches to a receptor. Once the cell is stimulated to act, the neurotransmitter is released. It is either reabsorbed by the nerve, called re-uptake, after which it is stored in a vesicle; or it is broken down by enzymes and excreted. If the process of enzymatic degradation or re-uptake and absorption did not occur, the cell receptors would be continuously stimulated (hyperexcited) or exhausted (desensitized).
The effects of drugs on the autonomic nervous system can be one of two impacts. The drug either increases the neurotransmitter’s ability to stimulate the cell’s receptors (agonist effect) or it blocks the cell’s ability to be stimulated (antagonist effect).
Figure 30-1 The cycle of neurotransmission.
Agonist drugs work by either increasing the amount of neurotransmitter (a direct effect) or decreasing the amount of re-uptake or enzymatic degradation, thereby indirectly increasing the amount of neurotransmitter. In both cases, these drugs would be considered an agonist.
Alternatively, a drug can act to block the neurotransmitter, and thus act as an antagonist. Drugs in this class work by either decreasing the amount of neurotransmitter, increasing enzymatic destruction of the neurotransmitter, or by competing with the neurotransmitter for the receptor, called competitive inhibition.
Cholinergic Receptors
Acetylcholine attaches to cholinergic receptors within the para-sympathetic nervous system. These cholinergic receptors can be further divided into muscarinic and nicotinic receptors.
Originally, the muscarinic receptors were identified for their affinity for muscarine, a poison found in mushrooms. Five different muscarinic receptors (M1-M2-M3-M4-M5) have been subsequently identified.15,16 For example, M2 receptors have been found in the cell wall of cardiac muscles.
Nicotinic receptors, the other cholinergic receptor, are located in the adrenal medulla, the central nervous system, and at many neuromuscular junctions, such as the muscles within the bronchioles. Similar to muscarinic receptors, nico-tinic receptors were identified for their affinity to nicotine. Blockage of the nicotinic receptors, an antagonist effect of drugs like pancuronium, results in smooth muscle paralysis, diaphragmatic paralysis, and respiratory arrest.
The main neurotransmitter that connects with either a muscarinic or nicotinic receptor is acetylcholine. Any chemical that mimics the action of acetylcholine (e.g., nicotine) is said to be a parasympathomimetic agent. Poison mushrooms often contain muscarinic-like chemicals that are cholinomimetic.17,18
Cholinergic Agents
The action of acetylcholine on the heart is to slow its rate through direct stimulation of the vagus nerve. Any drug which has a similar action, that mimics the effects of acetylcholine, would be called a cholinergic drug. Drugs of this sort usually have a subcomponent of acetylcholine, such as an ester or alkaloid-like molecule of acetylcholine, which binds directly to the cholinergic receptor. Pilocarpine is a drug in this classification.
Using an alternative mechanism to increase the amount of naturally occurring acetylcholine available, some drugs bind with the enzyme that breaks down the acetylcholine (acetylcholinesterase), thus rendering the enzyme inert. As a result, less acetylcholine is broken down and there is more acetylcholine available in the synaptic junction. An example of a drug that uses this mechanism is physostigmine, a drug used to treat open-angle glaucoma. Papillary constriction (miosis) is controlled by the parasympathetic nervous system. Physostigmine is also used to treat overdoses of atropine (whose action is discussed later) and tricyclic antidepressants; albeit rarely. Physostigmine is an example of a parasympathomimetic, a drug that mimics the action of the parasympathetic neurotransmitter acetylcholine.
Another anticholinesterase drug with parasympathomi-metic properties is neostigmine bromide. Neostigmine is used to help reverse the effects of certain neuromuscular blocking agents, called paralytics, which are used during emergencies to facilitate intubation.19
Anticholinergic Agents
Cholinergic blockers, those drugs that block acetylcholine from binding to either muscarinic or nicotinic receptors, are called anticholinergics. Drugs in this classification would stop parasympathetic activity.
Antimuscarinic drugs inhibit parasympathetic activity at the muscarinic receptors. Their greatest impact is on the core organs, such as the eyes, the gut, and the heart, because peripheral skeletal muscle primarily has nicotinic receptors.
An example of a muscarinic blocker is atropine sulfate. Atropine sulfate is a plant alkaloid derived from the deadly nightshade plant (latin—atropa belladonna)}9 Its fruit, a small black cherry, is poisonous. It was used, in small quantities, by Ladies of the Court in medieval Italy to add "brilliance" (pupil dilation) to their eyes; hence the name "bella donna" or beautiful lady. The name "atropine" comes from the Greek atropos, one of the Fates who held the shears to cut the thread of life. This reference is interesting in light of the fact that atropine is used to treat life-threatening bradycardia. Atropine’s effect is to block the vagus nerve (parasympathetic nerve) in the heart and reduce the vagal tone that slows the heart, causing the heart rate to rise.20
Because it is a parasympathetic blocker, atropine also decreases saliva production in the mouth, leaving the mouth dry (xerostomia). This effect is desirable prior to intubation. Atropine is also used as a pretreatment to prevent bradycardia induced by vagal stimulation of the hypopharynx, which is occasionally seen during pediatric intubation.
Atropine has received more interest lately as an antidote for certain nerve agents used as weapons of mass destruction. These nerve agents are structurally similar to the organophos-phate fertilizers. This treatment works by blocking parasym-pathetic receptors. Atropine is now available in auto-injectors for deep IM injection during an exposure to these deadly nerve gasses.21-24
The alternate anticholinergic is the nicotinic blocker. Nicotinic blockers have been used for decades in the operating room as a muscle relaxer. The earliest nicotinic blocker, curare, owes its origin to tribesmen in the equatorial Amazon. These tribesmen would easily bring down large animals, without killing them, by arrows that were dipped in curare. The animal was seemingly paralyzed and died from suffocation while still awake. In the 1850s, Claude Bernard showed that the South American Indian drug curare worked primarily at the neuromuscular junction and that a substance, later identified as acetylcholine, was blocked from receptors on the muscle cell. The idea that cells had receptors which could be affected by drugs had wide ranging implications for pharmaceutical research.20
Curare was a crude cholinergic blocker that was specific to the nicotinic receptors found in skeletal muscle. By blocking acetylcholine from attaching to nicotinic receptors on skeletal muscle, the muscles were, in effect, paralyzed. The advantages of a drug which could paralyze are numerous. For example, a paralyzed patient is easier to intubate and mechanically ventilate.25-27 Used together with sedatives and analgesics, these drugs have created an ideal intubation condition. Paralytics, as a class, do not cross the blood-brain barrier easily. Therefore, while the patient is paralyzed, he remains completely awake and sentient (sensing surroundings) and can experience feelings of pain. It is standard practice to co-administer a sedative and/or pain medication (analgesic) along with the paralytic agent to decrease the patient’s anxiety and relieve discomfort while paralyzed.
Some paralytics, particularly the early nondepolarizing agents, release histamine, a vasodilator, from the mast cells in the blood. Therefore, the patient’s blood pressure would fall. The next generation of paralytics (e.g., pancuronium) does not release histamine and therefore is more useful when treating patients at risk for hypotension, such as the trauma patient.
Depolarizing and Non-Depolarizing Neuromuscular Blockers
Neuromuscular blockers can be classified as either depolarizing or non-depolarizing. Depolarizing agents attach to the nicotinic receptor at the neuromuscular junction. In the resting state, the cell has charged sodium ions on the outside and potassium ions on the inside of the cell. This results in a difference in the electrical potential between the outside of the cell and the inside. This difference is called the resting membrane potential. With the nicotinic receptor stimulated by the drug, the cell opens the sodium channels in the cell wall and a rapid influx of sodium occurs. This results in depolarization and subsequently causes a cascade of events which then cause muscular contraction. These transient fine muscle contractions, seen after administration of a depolarizing neuromuscular blocker, are called fasciculations.
The depolarizing paralytic agent, however, remains bound to the receptor, unable to be broken down easily by the normal enzymes. This persistent action of depolarizing agents prevents the repolarization of the cell and a return of the cell to its normal resting state. Instead, the cell and the muscle remain flaccid (unable to be stimulated) and paralyzed.
As an alternative, non-depolarizing paralytic agents also bind with the nicotinic receptor but do not have the same effect on the cell. These agents simply bind to a receptor without causing depolarization. With the receptor site occupied, the cell remains in a ready resting state. However, the cell is unable to be stimulated because the receptor is blocked. This prevents the unwanted muscular fasciculations seen with depolarizing agents.
Adrenergic Neurotransmitters
Adrenergic neurotransmitters function in a manner similar to cholinergic neurotransmitters except that they act on the sympathetic nervous system. In the sympathetic nervous system nor-epinephrine, not acetylcholine, is the primary neurotransmitter.
Similar to the process in the parasympathetic nervous system, the sympathetic (adrenergic) nerve produces norepi-nephrine. To produce norepinephrine, the neuron takes the amino acid tyrosine and synthesizes it into dopamine, which is in turn converted into norepinephrine in the vesicles.
Norepinephrine is released from the vesicle, by an influx of calcium that occurs with neuronal stimulation, and floods the synapse between the nerve and the target cell. Attracted to adrenergic receptors on the cell wall membrane, the nor-epinephrine binds with the cell receptor and activates the enzyme adenyl cyclase, in a second messenger system, to convert adenosine triphosphate (ATP) into cyclic adenosine monophosphate (cAMP), releasing two phosphate molecules in the process. The two liberated phosphates are an energy-rich substrate which is used by many proteins within the cell to power metabolic processes (Figure 30-2).
After having caused the intended effect, the norepineph-rine is released from the receptor and may either diffuse into the general circulation or be taken up again by the adrenergic neuron.
The norepinephrine, assisted by an ATPase (enzyme), re-enters the neuron where it can either be stored in a vesicle or broken down by monoamine oxidase (enzyme) into inactive by-products (metabolites).
Adrenergic Drugs
It is important for the Paramedic to understand the sympathetic response because many drugs owe their therapeutic effect to the impact of these adrenergic drugs in the process of neurotransmission. For example, cocaine prevents the uptake of norepinephrine, thereby causing a buildup of norepineph-rine in the synapse, and hyperstimulation of the cell.28,29
Norepinephrine is an important neurotransmitter in the central nervous system (CNS). Inhibition of uptake of norepinephrine by tricyclic antidepressants, or the blockade of mono-amine oxidase (MAO) breakdown of norepinephrine by MAO inhibitors, can improve clinical depression, for example.30
There are two adrenergic receptors in the sympathetic nervous system: the alpha-receptors and the beta-receptors. These adrenergic receptors are further divided into alpha1 or alpha2 according to the organs on which they predominate (Figure 30-3). For example, beta1 receptors are found on cardiac muscle cells whereas beta2 receptors are found on arterial smooth muscle and bronchial smooth muscle.31-33
Adrenergic Agents
Several drugs have been created which mimic the effects of the sympathetic neurotransmitter norepinephrine. These drugs are called sympathomimetics. Sympathomimetics (often either prodrugs or analogs of norepinephrine) share a common base molecule, a catechol ring. Thus, they share a drug classification, and are called catecholamines.
Figure 30-2 Neurotransmission.
All catecholamines are very potent adrenergic agonists because they can cause a direct response from the adrener-gic receptor. In fact, most catecholamines, such as dopamine and epinephrine, have naturally occurring counterparts in the body. Because of this, the body also has a means of breaking down the drug more rapidly; in this case, with the enzymes monoamine oxidase (MAO) and another enzyme called catechol-O-methyltransferase (COMT). In order to maintain a therapeutic effect, these drugs are continuously infused intravenously. The infusion is then slowed, a process called weaning, to the point where the body is able to sustain itself and is no longer dependent on the infusion to maintain vital functions such as blood pressure.
Realizing the limitations of catecholamines, newer non-catecholamine compounds have been created (i.e., those without catechol ring). Without the ring, the enzymes COMT and MAO have more difficulty neutralizing the drug into an inactive metabolite. Therefore, these drugs enjoy a longer duration of action. Ephedrine, a common ingredient in decongestants, is an example.
Adrenergic agonists may work by direct action or by indirect action. Direct acting adrenergic agonists couple with and excite the adrenergic receptors. The indirect agonists cause the release of norepinephrine from the terminal neuron, which in turn causes the norepinephrine to attach to the adrenergic receptors and the receptors to react.
The five direct-acting adrenergic agonists— norepinephrine, epinephrine, dopamine, dobutamine, and isoproterenol—all have similar effects on the cardiovascular system.34 These drugs elevate blood pressure and thus are also called vasopressors. However, each drug has an action that is slightly different from the others, thus making one more desirable than another for different circumstances.
The indirect adrenergic agonists are stimulants that cause the release of norepinephrine. An example ofan indirect adrenergic agonist is the class of drugs called amphetamines.
Systemic Pharmacologic Effect
Owing to the widespread distribution of adrenergic receptors in the major core organs, and the often dramatic effect these sympathomimetic drugs can have upon the sympathetic nervous system, a review of systems will be discussed.
The heart may be the organ system most affected by adrenergic agonists. These powerful medicines can markedly increase the heart’s strength of contraction (positive inotro-pic effect) as well as rate (positive chronotropic effect) as a result of increased calcium influx in the myocardium. The calcium influx causes a higher action potential and quicker depolarizations. Left alone, stronger, faster contractions lead to more complete ventricular emptying and an increased cardiac output.
Catecholamines, such as epinephrine, can be potent cardiac stimulators. This is the anticipated action of epinephrine during a cardiac arrest. Epinephrine should either (1) increase the fibrillatory action of the arrested heart, coarsening the ventricular fibrillation, so that subsequent defibrillations are more likely to be successful, or (2) induce spontaneous pacemaker activity in the heart in cardiac standstill (asystole).
Conversely, catecholamines like epinephrine, when inappropriately administered, can induce spontaneous depolarizations and extra-systoles by the same mechanisms. These actions can disrupt a normal cardiac sequence and send the heart into chaos and ventricular fibrillation.
The peripheral capillary beds are largely controlled by alpha1 receptors of the sympathetic nervous system as well.35,36 During times of stress, when blood is needed in the core organs, these capillary beds can be preferentially shut down. Blood will then be directed toward the body core in a process called shunting. This shutdown of capillary beds also causes a higher total peripheral vascular resistance (PVR) to forward blood flow.
In certain abnormal perfusion states, such as anaphylactic shock or septic shock, peripheral vascular resistance is reduced. Then catecholamines, such as dopamine or norepinephrine, can help to restore a higher PVR.
Administration of catecholamines, such as dopamine, can result in a higher PVR through alpha1 receptor stimulation. While dopamine is a very useful drug when used appropriately, a high PVR presents an obstacle to forward blood flow from the heart (i.e., cardiac output). This effect can create such a large demand on the heart’s muscle that it cannot keep up with demand and the patient may manifest symptoms such as chest pain (angina) and possibly sustain a myocardial infarction.
Figure 30-3 The effects of adrenergic receptor stimulation.
The differences between the effects produced by each of the catecholamines, and even the differences between the effects of a single catecholamine at different doses, requires careful monitoring of the patient for untoward effects.
The smooth muscles of the bronchi and the bronchioles are also richly supplied with beta2 receptors and are easily affected by catecholamines. When activated, these receptors cause dilation of medium-sized airways. This positive effect is so pronounced that these agents have become a mainstay in the treatment of bronchoconstriction and are discussed in more detail in the section on drugs that affect the respiratory system.
One of the notable effects of catecholamines on the endocrine system is the increase in the amount of blood glucose available to be used as energy. This is achieved through a combination of decreased insulin secretion from the pancreas and increased breakdown of glucagon in the liver and muscles. The intended effect of the elevated blood glucose levels is to have more fuel for an active metabolism during an emergency.
STREET SMART
Topically applied, epinephrine and epinephrine derivatives such as phenylephrine can create a localized vasoconstriction in exposed capillary beds in the mucosa. A solution sprayed into the nasal mucosa of the nostril prior to intubation can reduce the likelihood of bleeding during a nasal intubation.37 38 The key to successful use of these agents is to apply them before other lubricating substances and in time for the medication to take the desired effect. Upon quick visualization, the mucosa should appear pale after vasoconstriction has occurred.