Introduction
The fundamental problem in addressing prion diseases, or the transmissible spongiform encephalopathies, is finding an explanation for the massive neu-ronal death that occurs. Although some understanding of the mechanism by which neuronal death occurs comes from studies with scrapie-infected mice, most of the insights regarding a possible mechanism have come from cell culture models in which a synthetic peptide (PrP106-126), based on the sequence of the prion protein, has been applied to neuronal cells. This review describes the details of the mechanism of toxicity of this peptide to cells.
Prion Diseases
Prion diseases are characterized by neuronal death or neurodegeneration. However, prion diseases are more widely known because of the theory that infectivity is carried by a protein only, namely the abnormal isoform of the prion protein (1). Despite this controversial and interesting claim, it is probably only relevant to a small proportion of human sufferers of these diseases. The vast majority of cases of human prion disease are Creutzeldt-Jakob disease (CJD), which occurs spontaneously with no known cause (2). There are also inherited forms of prion disease, which include Gerstman-Straussler-Scheinker syndrome, fatal familial insomnia, and inherited CJD (2,3). The number of cases in which transmission of disease has occurred by infection is quite limited. The only confirmed cases are those of iatrogenic transmission resulting from transplantation of human tissue, such as dura mater or central nervous system products (4). The disease, Kuru, is believed to have been spread by eating of human brains. Although there are a number of unequivocal similarities between bovine spongiform encephalopathy (BSE) and the recently described new variant CJD (nvCJD) (5,6), a causal connection between the two diseases has not been demonstrated.
The human diseases are linked together collectively with several animal diseases, which include BSE of cattle (7), scrapie of sheep (1), chronic wasting disease of deer (8) and transmissible mink encephalopathy (9), because large amounts of an abnormally folded isoform of the prion protein (PrPSc) can be detected within the brains of affected individuals (2). PrPSc is a derivative of a normal extracellular glycoprotein termed "cellular prion protein" (PrPC). Although widely accepted as being the "prion" or infectious agent of prion disease, PrPSc, generated by the host, may not be the only component of the infectious agent. Despite this uncertainty, there is little doubt that accumulation of host-generated PrPSc is the cause of neurodegeneration in these diseases. Nevertheless, the mechanism by which PrPSc is causative to the neurodegeneration remains unresolved.
PrPC has been shown to be a copper-binding protein (10), which influences uptake of copper (11), and also functions as an antioxidant (12) which protects cells from oxidative stress. Mice deficient in expression of PrPC have reduced activity of the cytoplasmic superoxide dismutase (SOD-1) (13), probably because of reduced incorporation of copper into SOD-1 (14) by a number of different cell types. Given that the protein has a rather general function, it is not surprising that it is expressed by a number of different cell types, including neurons (15), astrocytes (16,17), microglia (18), muscle cells (19), keratinocytes (20), and various cells of the blood (21). Nevertheless, the highest level of expression is within the nervous system at endplates and more particularly at synapses in the CNS. Despite PrPSc accumulation in other tissues in prion disease (22), it is probably because of this neuronal expression that most PrPSc accumulates in the central nervous system.
The primary hypothesis concerning the nature of neuronal death in prion disease is that PrPSc is directly neurotoxic. The chief competing hypothesis is that, in conversion of PrPC to PrPSc, the normal function of PrPC is lost. This loss of function would then lead to a deterioration in normal cellular metabolism, which would trigger apoptosis in neurons. This theory, as a stand alone description of the cause of neurodegeneration, has been dismissed by studies with mice lacking PrPC expression (23). Such mice live normally and show no signs of prion disease. It is still possible that another molecule functions in place of PrPC, or that the copper metabolism and/or the oxidative metabolism of PrPC-deficient cells adjusts to deal with the lack of expression. Nevertheless, work with cultured neuronal cells indicates that loss of PrPC function probably is involved in the mechanism of cell death. This mechanism is described in detail below.
Neurotoxicity of PrPSc In Vivo
The principal model of study of prion disease is artificial infection of mice or hamsters with the scrapie agent of sheep. Despite 20 years or more of study of scrapie in rodents, few details have emerged to explain the mechanism behind neurodegeneration in prion disease. Neurons undergo apoptosis at an accelerating rate toward the end of the disease, as symptoms begin to develop before death (24,25). However, it is not possible to conclude from these studies that cell suicide is responsible for all of the cell death that occurs.
Progression of cell death and its time-course have been studied in detail (25-27). The occurrence of neuronal loss reflects quite closely the distribution of PrPSc. However, this distribution and the extent and form of the resulting pathology is variable, and depends on the strain of scrapie agent used to initially infect the rodents (28). Some aspects have been clearly demonstrated by a number of groups. First, microglia present in the vicinity of PrPSc become activated before symptoms or any detectable neuronal death (24,25,29). Second, astrocytosis begins at about the same time that neuronal death begins and is a consistent feature of the disease. Other changes have also been noted such as increases in various cytokines (30), evidence of oxidative damage, and decrease in activity of nitric oxide synthase (31). However, the causal connection between any of these changes and neuronal death is unclear.
Furthermore, evidence is missing for PrPSc being directly toxic in vivo. To date no one has managed to inject PrPSc or a derivative of the protein into an animal and observed acute neuronal loss. A recent investigation claims that the amount of PrPSc needed to induce disease in mice by direct intracerebral injection is not sufficient to produce local PrPSc-specific neurodegeneration (32). The effect may have been masked by cell death induced by the surgical procedure. However, the implication of this, if true, is that, to be neurotoxic PrPSc must be generated by the host.
A further observation is that accumulation of large amounts of host-generated PrPSc is also not sufficient to cause neurodegeneration. PrPC-knockout mice (23), that had received grafts of neural tissue overexpressing PrPC showed accumulation of PrPSc in PrPC-deficient tissue (i.e., of their brains), following infection with the scrapie agent, but neurodegeneration was not detectable in the vicinity of this accumulation (33). This suggests that PrPC expression by the host is necessary for PrPSc-induced neurodegeneration.
Although host expression of PrPC and host generation of PrPSc appear to be necessary to a toxic model of neurodegeneration, neuronal expression of PrPC may not be necessary. Transgenic mice that express PrPC only in astrocytes have been generated (34). Such mice do not express PrPCin neurones, and all PrPSc generated by such mice upon infection with the scrapie agent must be produced by astrocytes. These can succumb to prion disease and show vast neurodegeneration. This implies that neurodegeneration in prion disease may be caused by PrPC-expressing astrocytes (34). However, in these experiments in which there is no neuronal expression of the prion protein, there is also no function of the prion protein in neurons, and the possible causal involvement of lack of neuronal PrPC function in the neurodegeneration in these mice is unknown.
Recent experimental evidence using transgenic mice expressing a truncated prion protein of 106 amino acids sheds light on what part of the prion protein molecule is necessary for both infection and neurodegeneration (35). The truncated version of PrPC expressed by these mice can be converted into a truncated PrPSc capable of infecting the same transgenic mice, and inducing neurodegeneration. The 106 amino acids comprise the amino residues 89-140 and from 177 to the C-terminus. Other work has implicated the C-terminus of the molecule in conversion of the PrPC to the abnormal isoform and possible interactions with chaperone molecules, which may aid conversion (36,37). This is the strongest evidence so far that the region of the protein around amino residues 89-140 is necessary for the toxicity of PrPSc.
Neurotoxicity In Vitro
Further elucidation of the requirements and mechanism of toxicity of host-generated PrPSc has been found to be difficult in vivo, because of the inability to separate requirements for infectivity and conversion of PrPC to PrPSc from those of the resulting neurodegeneration. Most attempts at understanding PrPSc -induced neurodegeneration have been based on cell culture studies, which have demonstrated that PrPSc application to neuronal cells results in a toxic effect (24,38). Use of the full length molecule is difficult, because no true pure source of PrPSc exists. For such studies PrPSc is derived from extracts of brains from mice treated with proteinase K. Furthermore, no work with recombinant PrPC has managed to generate PrPSc. Although there are many claims of in vitro conversion (39), these studies either require addition of large amounts of PrPSc or produce protease-resistant protein that does not appear to be infectious, and only generates multimers and not true fibrils (40,41).
In an attempt to generate pure protein that mimics PrPSc, researchers have turned to the use of synthetic peptides. Forloni et al. (42) were the first to examine the neurotoxicity of PrP to try to identify the toxic domain (amino residues 89-145). They synthesized distinct peptides, based on portions of the human prion protein sequence and demonstrated that one of these, PrP106-126, was fibrillogenic, protease-resistant, and induced neuronal death by apoptosis in cultured hippocampal cells. The parallels between this peptide’s structure and that of PrPSc mean that it is reliable as a synthetic model of the whole protein, and has been extensively used as such by a large number of groups.
Table 1
Summary of Peptide Qualities
|
Peptide |
Fibril formation |
Toxicity |
Requirements |
|
|
|
|
Microglia |
PrPC |
|
|
PrP59-66 |
No |
na |
na |
|
|
PrP59-91 |
No |
- |
na |
na |
|
PrP89-106 |
No |
- |
na |
na |
|
PrP106-126 |
Yes |
++ |
Yes |
Yes |
|
PrP112-126 |
Yes |
+++ |
Yes |
Yes |
|
PrP113-134 |
Yes |
++ |
Yes |
Yes |
|
PrP113-116 (AGAA) |
No |
- |
na |
na |
|
PrP113-120 (AGAAAAGA) |
Yes |
- |
na |
na |
|
PrP121-134 |
No |
- |
na |
na |
|
PrP127-147 |
Minor |
-/+ |
No |
No |
|
PrPSc |
Yes |
++++ |
Yes |
Yes |
Shown are all peptides that have been tested for toxicity. This table summarizes results from various sources (24,42,43,49,50,54,71). The results for the different peptides are compared to those known for PrPSc. PrPxxx-xxx indicates the amino residues of the human PrPC sequence on which the synthetic peptide was based. Under "Requirements," PrPC indicates need for neuronal PrPC expression for toxicity; "Microglia indicates" that reduction in microglia content abolishes toxicity. na = not applicable. Number of + under toxicity indicates magnitude of toxicity.
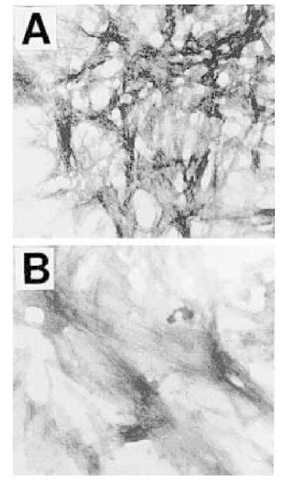
Recently, a reevaluation of the toxicity of PrP106-126 has been carried out (43). Other peptides, generated around the region of 106-147, were tested to determine the smallest derivative of the human prion protein that mimics the toxic mechanism of PrPSc with maximal toxicity. A summary of the toxicities of various PrP peptides is shown in Table 1. Some peptides, such as PrP127-147 or PrP121-134, show little or no toxicity. The peptide PrP113-120, was also found to be not toxic, however, this peptide was found to form fibrils (Fig. 1) and is currently the shortest known fibrillogenic peptide with the palindromic sequence, AGAAAAGA. Additionally, although this peptide was not toxic on its own, it was necessary for the toxicity of larger peptides, such as PrP106-126. The most toxic peptide sequence tested was that of PrP112-126, which peptide corresponds to a region of the protein conserved entirely in every known species in which the prion protein has been sequenced (44,45). The findings indicate the importance of the AGAAAAGA region of the prion protein. A peptide based on this region of the protein can also be used to inhibit toxicity of PrP106-126 (43).
Fig. 1. Fibrils of PrP. Electron microscopic photomicrographs of peptides prepared in phosphate-buffered saline and stained with PTA to produce shadowing. (A) PrP106-126. (B) AGAAAAGA. These findings make the peptide AGAAAAGA the shortest known fibrillar peptide. Magnification was x50,000.
Furthermore, deletion of this region of PrPC in transfected cells prevents the formation of PrPSc (46). Peptides generated which include the AGAAAAGA region, inhibit in vitro conversion of PrPC to PrPSc, suggesting that these amino residues are essential for the interaction of the two molecules necessary for the conversion event (47). From these results, it is likely that this region of the protein will emerge as being the essential component of PrP’s protein sequence, regarding both conversion to the abnormal isoform and neurotoxic nature of PrPSc.
PrP106-126 causes apoptosis of neurons in cultures derived from mouse brain (42). Before describing what is known about the mechanism of this effect, it is perhaps necessary to point out what kinds of cells are susceptible to this toxicity. However, it is also necessary to point out that the toxicity of PrP106-126 to any cell appears to be dependent on the presence of an additional factor in the form of some kind of stress such as oxidative stress. PrP106-126 is not toxic to pure populations of neuronal cells. Neuron-like cells of various cell lines, such as neuroblastomas (N2A) or PC12 cells (48), are not susceptible to the toxicity of PrP106-126 on their own. Several tumors of neuroectodermal origin have been studied, in addition, but these cells are only susceptible to PrP106-126 toxicity if they possess a mixed phenotype (D. R. Brown, unpublished observations). The presence of a source of oxidative stress, such as can be produced by activated microglia, seems to be a critical factor (but not sufficient) for PrP106-126 toxicity.
Under appropriate conditions PrP106-126 is toxic to neurons from the hippocampus, cerebellum and neocortex (42,49,50). The peptide is also toxic to myotubes (19), nerve growth factor-differentiated PC12 cells or PC12 cells expressing high levels of PrPC (48). In the presence of an inhibitor of cell division, PrP106-126 is also toxic to other cells, such as astrocytes (51) and microglia (52). Susceptibility to PrP106-126 toxicity appears to depend on two conditions: only cells expressing relatively high levels of PrPC are susceptible, and, of these, only, or mostly, nondividing cells are susceptible.
Mice deficient in PrPC expression cannot be infected with PrPSc (53). As indicated above, accumulation of PrPSc in PrPC-deficient tissue does not result in toxicity to neurones (33). However, direct injection of PrPSc into the brain of PrPC-expressing (wild-type) mice also does not induce neurodegeneration (32). Analysis of cerebellar cells and cortical cells from these mice also suggests that the PrP106-126 peptide was not toxic to neuronal cells lacking PrPC-expression (49,54). This effect does not result from failure of PrP106-126 to enter neurons. Studies with biotinylated peptide have shown that PrP106-126 enters the same neuronal compartments in PrPC-deficient neurons as PrPC-expressing neurons (55). The lack of toxicity to PrPC-deficient cells is not caused by greater resistance of these cells to toxic substances. In fact, PrPC-deficient cells are more susceptible to apoptosis-inducing substances, and to oxidative stress, than are PrPC-expressing neurons (13,54). The reason why PrP106-126 (or PrPSc) addition to PrPC-deficient cells does lead to increased cell death is presently unknown. However, it is possible that binding of the peptide, either to a receptor (56), upregulated with PrPC expression, or to PrPC itself, may be necessary for activating the appropriate signal transduction cascade within neurons.
Although there is some evidence that toxicity of PrP106-126 increases with increased levels of PrPC expression (57), the relationship is not that clear. Studies were also carried out with mice overexpressing PrPC. However, PrP106-126 was only more toxic to cerebellar cells from one strain of overexpressing mice (Tg35) than to wild-type cells but was not more toxic to cells from another strain (Tg20) (58). The increased toxicity to the Tg35-over expressing cerebellar cells was related to changes in the behavior of microglia in the cultures, and not in the metabolism of the neurons. Therefore, above a certain critical level of expression, increasing PrPC at even higher levels may not increase susceptibility to toxicity.
A Role for Microglia
Evidence for a role for microglia in the toxicity of PrP106-126 to neuronal cultures came from two kinds of experiments. Cultures of wild-type (PrPC-expressing) cerebellar cells were treated with L-leucine methyl ester, a compound that is selectively toxic to microglia. This treatment abolished toxicity of the peptide (54). At about 4 d in culture (and 4 d of peptide treatment), cerebellar cell cultures consist of approx 85% neuronal cells, 10% astrocytes and 5% microglia. The L-leucine methyl ester treatment reduces microglia content by 80% (D. R. Brown, unpublished observations). Second cerebellar cell cultures treated to remove microglia were co-cultured with increasing numbers of microglia. Increasing microglia content increased the toxicity of PrP106-126 to cerebellar cell cultures (54).
Such observations on their own, would suggest that PrP106-126 toxicity is indirect and a result of toxic factors released by activated microglia. However, co-culture of wild-type microglia with PrPC-deficient cerebellar cells did not result in PrP106-126 toxicity to PrPC-deficient cerebellar neurons (54). PrPC expression remained the principal necessity for PrP106-126 toxicity. Nevertheless, microglia in these cultures were necessary for the visible toxic effect.
The toxic agent released by the microglia was found to be superoxide (or reaction products) (54). Other possible toxic agents released by microglia, such as nitric oxide or tumor necrosis factor were found not to be involved (D. R. Brown, unpublished observations). The necessary quality of microglia in the toxic mechanism of PrP106-126 could be replaced by an alternative source of superoxide, such as xanthine oxidase, an enzyme that generates superoxide. Microglial-reduced cultures of cerebellar cells, incubated with PrP106-126 and xanthine oxidase, lead to PrP106-126 toxicity to the cerebellar cells (54). Thus, for PrP106-126 to be toxic to PrPC-expressing neurons, the neurons must be concomitantly exposed to a source of oxidative stress. In support of this it has been found that inhibitors of oxidative stress or antioxidants can inhibit the toxicity of PrP106-126 (54).
The effect of PrP106-126 on microglia has also been examined. The peptide induces proliferation of microglia, as well as activation (52,59). Although activated microglia are inhibited from proliferating, it is probable that two sub-populations respond differently to the peptide. Activation of the microglia is accompanied by release of calcium from intracellular stores (52), and possibly uptake through L-type channels (60). However, the latter may be related to death commitment, because PrP106-126 was found to be toxic to a small sub-population of microglia, when proliferation was inhibited with cytosine arabi-noside (52). PrP106-126 also induces release of the cytokines, interleukin-6 (IL-6) and IL-1 (61), from microglia which are probably related to induction of astrocyte proliferation (51). However, there has been no further reliable analysis of the mechanism by which PrP106-126 activates microglia.
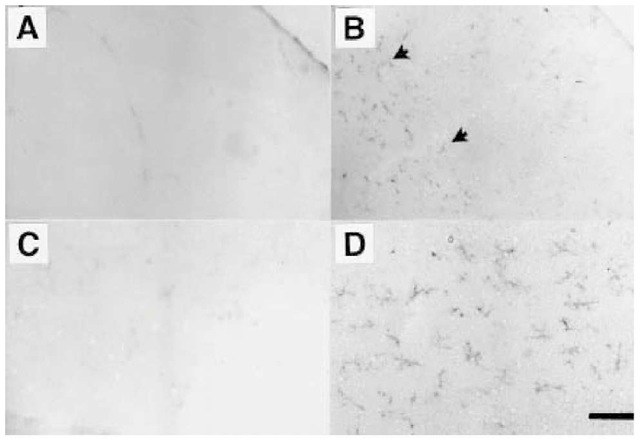
Microglia also express PrPC (18). The level of microglia expression alters the ability of astrocytes to be activated by substances such as endotoxins (18). Cultures from one particular strain of PrPC-overexpressing mice (Tg35) could be easily activated by lipopolysaccahride whereas activation of microglia from PrPC-deficient mice resulted in little superoxide release. The reasons for this remain unclear. Analysis of PrPC-expression in the overexpressing microglia revealed a difference in the level and glycoforms of PrPC expressed (58). As well as being more easily activated, the microglia in Tg35 mice proliferated more rapidly both in culture and in vivo. Staining of microglia in sections of cerebellum from wild type and PrPC overexpressing mice showed higher levels of Mac-1 positive microglia (Fig. 2). These Tg35 mice show paralysis and neurological changes as they age, which may be related to higher numbers of activated microglia in their brains.
The relevance of microglial activation to prion disease was confirmed by studies of scrapie-infected mice. Large numbers of activated microglia have been detected in the brains of scrapie infected mice long before any clinical changes can be detected (29). These changes in microglia occur concurrent with increased accumulation of PrPSc (24). However, the changes that occur as the disease progresses are complex and assigning a causal role to microglia from such data would be presumptive.
A Role for Astrocytes
Apart from neurodegeneration, the other major change that occurs in prion disease is gliosis, and especially astrogliosis. Increased glial fibrillary acidic protein (GFAP) has been used as a marker for astrogliosis in prion disease. Levels of GFAP increase continuously in all brain regions after PrPSc is detected (62). The astrogliosis is typical of the end stage of the disease, and is closely associated with the increased neurodegeneration observed. However, although an environment containing activated astrocytes can be deleterious to neuronal survival, it is unclear whether astrogliosis stimulates neurodegeneration in prion disease.
As with neurotoxicity, application of PrP106-126 to astrocytes in culture has been used to model astrogliosis in vitro. PrP106-126 stimulates astrocyte proliferation and microglia proliferation in mixed glial cultures (59). However, PrP106-126 did not stimulate astrocytes to proliferate in the absence of microglia.
Fig. 2. Microglia. Photomicrographs of sections from adult mouse cerebellum (4-5 mo of age). Wild-type (A and C) and Tg35 (B and D) were stained with an antibody to Mac-1. Microglia stained with Mac-1 appear darkly colored (arrowed). No staining was observed in wild-type sections, but large numbers of microglia are present in Tg35 sections. The stained in A is enlarged in C, and that of B is enlarged in D. Scale bar = 400 \xM in A and B and 100 \xM in C and D.
Pure cultures of astrocytes did not show increased proliferation when PrP106-126 was added (59). However, the presence of microglia alone was not sufficient for PrP106-126 to induce astroglial proliferation. Like microglia and neurons, astrocytes express PrPCin culture (17). PrPC-deficient astrocytes did not proliferate in the presence of both PrP106-126 and wild-type astrocytes (51). Therefore, astrocytic PrPC-expression is necessary for PrP106-126 induced astrocytic proliferation. Furthermore, conditioned media, from micro-glia treated with PrP106-126, was insufficient to induce astrocytic proliferation (18). Similarly, conditioned medium from microglia treated with granulocyte-macrophage-colony-stimulating factor (GM-CSF), a mitogen of microglia, had only a minor effect on astrocytic proliferation, implying that the proliferation of astrocytes induced by PrP106-126 in the presence of microglia, is not simply a result of cytokines released by microglia. However, conditioned medium from GM-CSF-stimulated microglia did greatly enhance the proliferation of astrocytes in the presence of PrP106-126. This implies that PrP106-126 primes astrocytes to be more sensitive to stimulation from cytokines, such as IL-1 and IL-6, released by microglia. This implies that PrP106-126 also has a direct effect on astrocytes necessary for stimulation of astrocytic proliferation.
PrP106-126 has other direct effects on astrocytes. PrP106-126 has been shown to inhibit the uptake of glutamate (63), an important neurotransmitter in the central nervous system. Clearance of glutamate after its release at synapses is necessary to protect neurons from the excitotoxic effects of glutamate. Thus, inhibition of glutamate uptake by astrocytes may be another way that PrP106-126 could indirectly cause neurotoxicity.
The role of astrocytes in the neurotoxicity of PrP106-126 to cerebellar cell cultures has been assessed. Treatment of cerebellar cells with a-aminoadipic acid, a substance that selectively kills type 1 astrocytes (64), does have a minor effect in inhibiting PrP106-126 neurotoxicity (17), but not enough for this to be a major contributing factor in the toxicity of PrP106-126 to this system. For freshly prepared cerebellar cell cultures on their own, the involvement of astrocytes in PrP106-126 toxicity was dismissed (54).
Recent studies (65,66) have advanced understanding of the interaction of neurons with large numbers of astrocytes which is more typical of later stages of scrapie, following gliosis. In the presence of large numbers of astrocytes, cerebellar neurons become dependent on astrocytes for protection from glutamate toxicity. Coculture of neurons with astrocytes increases the velocity of uptake of glutamate by astrocytes. The coupling of astrocytes and neurons in culture, in this manner results in an increased susceptibility of neurons to the toxicity of glutamate, but, in the presence of astrocytes actively clearing glutamate this has little consequence. This increased sensitivity to glutamate of neurons may represent an enhanced efficacy of glutamatergic synapses. However, an in vivo correlate of this is still unknown. The implication for glutamate toxicity is that any substance that activates astrocytes, or inhibits their ability to clear glutamate, will expose neurons to the toxicity of glutamate.
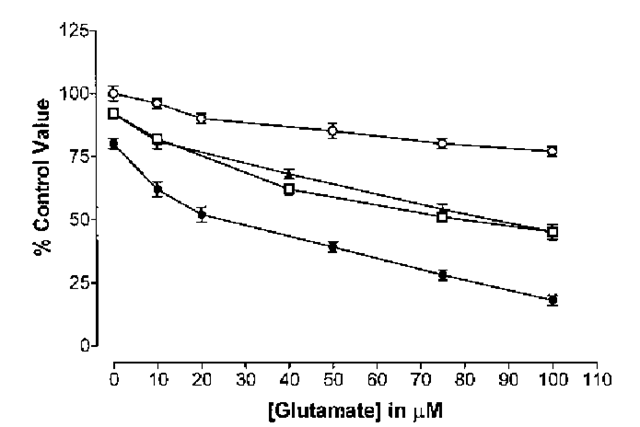
PrP106-126 is not toxic to neurons deficient in PrPC (49) and PrP106-126 only inhibits glutamate uptake by PrPC-expressing astrocytes (63). A coculture system between PrPC-deficient neurons and PrPC-expressing astrocytes was prepared. In such a system micromolar concentrations of glutamate were found not to be toxic to PrPC-deficient neurons, although inhibition of astrocytic glutamate clearance by PrP106-126 caused neuronal loss because of glutamate toxicity (17). However, the concentration of glutamate present in the culture during such experiments was not sufficient to kill PrPC-deficient neurons in the presence of PrP106-126 without astrocytic co-culture. In this system, the dependence of PrPC-deficient neurons on astrocytes, for protection from glutamate toxicity, meant that inhibition of glutamate uptake by PrP106-126 stripped the PrPC-deficient neurons of their protection, and resulted in a glutamate induced toxic effect. Thus, in this system, PrP106-126 can have an indirect toxic effect on neurons by interfering with neuron-astrocyte interactions (Fig. 3).
Fig. 3. PrP106-126 is not toxic to PrP-deficient cerebellar cells. PrP-deficient cer-ebellar cells were cultured alone or co-cultured with wild-type (PrP-expressing) astro-cytes. The cells were exposed to increasing concentrations of glutamate with or without addition of 80 ^M PrP106-126. After 4 d of treatment, the survival of the PrP-defi-cient cerebellar cells was determined by carrying out an MTT assay on the cerebellar cells only. Co-culture with wild-type astrocytes (O) significantly (Student’s t-test, p < 0.05) reduced glutamate toxicity, compared to the toxicity of glutamate to cerebellar cells not co-cultured (D).PrP106-126 did not enhance the toxicity of glutamate to cerebellar cells not co-cultured (A). However, PrP106-126 greatly enhanced the toxicity of glutamate to cerebellar cells co-cultured with astrocytes (•). PrP106-126 enhanced glutamate toxicity at all concentrations and dramatically abolished the protective effects of astrocytes on protection of neurons against glutamate toxicity. The toxic effect of the glutamate in the presence of PrP106-126 and astrocytes was greater than if the astrocytes had not been present.
It is possible that these results only represent a curious cell culture effect. However, there are reasons to suggest that this is not the case. Mice have been generated that expressed PrPC via a GFAP promoter (Tg3/Prnpo/o mice) (34). In these mice, only astrocytes (and not neurons) express PrPC. After infection with the scrapie agent, these mice developed neurodegeneration and accumulation of PrPSc similar to prion disease. It is possible the neurodegeneration seen following scrapie infection of these mice, which is similar to that seen in prion disease, comes about either wholly or in part as the result of glutamate toxicity. Indeed, experiments using the mice described in Raeber et al. (34) supports this (17). PrP106-126 was only toxic to these cultures when excess Tg3/ Prnpo/o astrocytes (which are Prnp+/+) were added to cerebellar cell cultures from Tg3/Prnpo/o mice. It is likely that PrP106-126 was not toxic to cerebellar cell cultures without co-culture, because there were insufficient numbers of astro-cytes present. This confirms that PrP106-126 can only be toxic to PrPC-deficient neurons in the presence of excess numbers of PrPC-expressing astrocytes. Previous work on the phenotype of PrPC-deficient neurons suggests that they are compromised in their ability to resist oxidative stress (13). PrP106-126 is known to have effects on PrPC-deficient neurons that also alter their resistance to oxidative stress (13,54). The neurons in the mice of Raeber et al. (34) are PrPC-deficient. As PrP106-126 alters the wild-type neuronal phenotype to one similar to that of a PrPC-deficient neuron then possibly there is no contradiction between their findings and that of Brandner et al. (33), who suggest that PrPC expression is necessary for the neurodegeneration seen in prion disease. By the late stages of prion disease neurons may be devoid of functional PrPC because of conversion to PrPSc, and may be phenotypically PrPC-deficient.
Collectively, these observations imply a complex picture of the toxicity of PrP106-126 or PrPSc. However, they also emphasize the likely role played by glia in toxicity. Microglia may play a role in initiating indirect aspects of neu-rotoxicity, and also in inducing astrocyte proliferation. Neurodegeneration may also be accelerated by astrogliosis, and complex interactions between neurons and astrocytes may imply that the massive round of apoptosis occurring at the late stages in the disease are a result of glutamate toxicity. Inhibiting the response of N-methyl-D-aspartate receptors may thus prove to be beneficial in slowing disease progress.