Introduction
Various approaches have been taken to study the function of prion proteins. Biochemical methods were applied to search for a binding partner of PrPC which is attached to the cell surface by a glycosylphosphatidylinositol GPI anchor (1). The glial fibrillary acidic protein was one of the first possible binding partners to be described (2) followed by Bcl-2 (3,4), molecular chaperones (5), amyloid precursor-like protein 1 (6), the 37-kDa laminin receptor (7) and a 66-kDa membrane protein which has not been characterized in more detail (8). However, it has not been possible to show any biological significance for PrPC binding of these proteins. Based on biochemical analyses of chicken PrPC, Harris et al. (9) hypothesized that PrPC may play a role in the regulation of the expression of cholinergic receptors at the neuromuscular endplate.
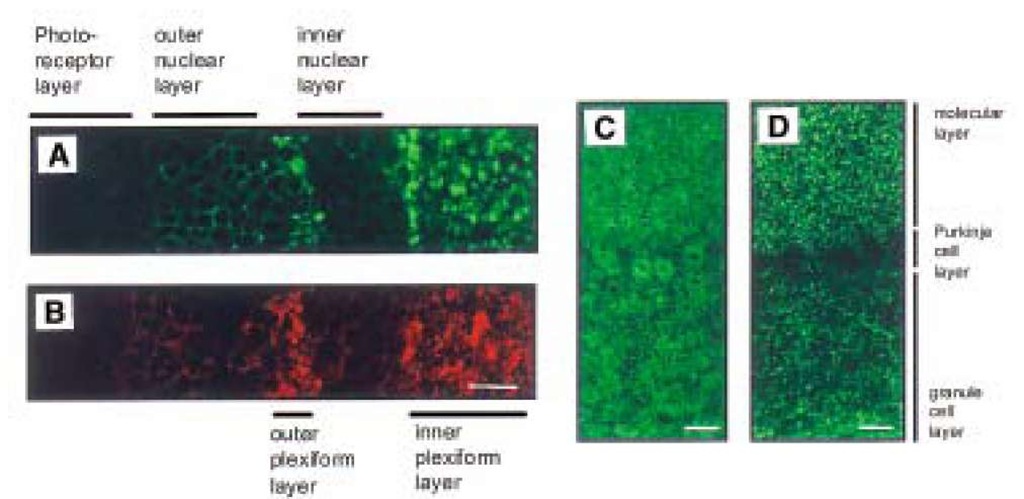
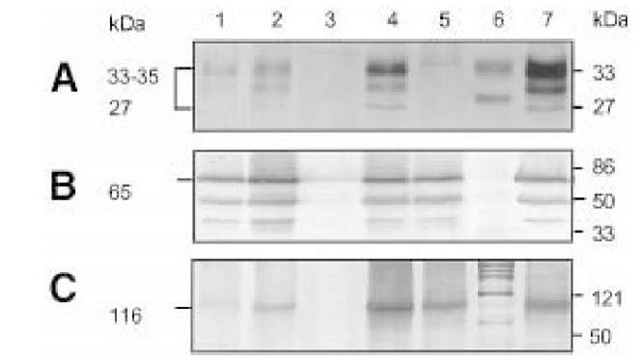
Biochemical, morphological, and electrophysiological studies of the first PrP gene (Prnp) knockout mouse (Prnp0/0 mouse), which was generated by Bueler et al. (10), showed a regular expression of the acetylcholine receptor (11). Except for changes in its circadian rhythm (12,13) and increased sensitivity to seizures (14), this Prnp0/0 mouse showed no developmental or behavioral changes (10). These findings were confirmed in studies of another Prnp0/0 line generated by Manson et al. (15). The lack of severe defects in these two lines of Prnp0/0 mice was ascribed to adaptation, because PrPC was absent throughout embryogenesis. However, transgenic mice expressing inducible PrPC- transgenes that were rendered PrPC-deficient as adults by administration of doxycycline have remained healthy for more than 1.5 yr (16). A third Prnp0/0 mouse generated by Sakaguchi et al. (17) showed progressive ataxia and loss of Purkinje cells in mice aged more than 70 wk. Also, a fourth independently generated Prnp0/0 mouse (18,19) exhibits ataxia and Purkinje cell degeneration. Weissmann (20) suggested that additional deletions of intronic sequences of Prnp may play a role in this knockout line. Most recently the upregulation of a novel PrPC-like protein, designated Doppel, whose gene is located 16 kb downstream of the mouse PrP, has been speculated to be the cause of Purkinje cell degeneration observed in two of the Prnp0/0 mouse lines (21). Even though the hypothesis of the interaction of prion proteins with cholinergic receptors thus could not be confirmed, the studies of Harris et al. (9) indicated that PrPC is enriched at the neuromuscular end-plate, i.e. at synaptic endings. Indeed immunohistochemistry of PrPC-overexpressing transgenic mice reveal a synaptic expression pattern of PrPC (22,23). PrPC is predominantly expressed in regions of high synaptic density, such as the inner and outer plexiform layer of the retina or the cerebellar molecular layer (Fig. 1), in contrast to earlier studies in which a predominantly somatic expression of PrPC was described (24-26). Further evidence for a preferentially synaptic location of the prion protein in the central nervous system was shown in immunoelectron microscopic studies by Fournier et al. (27) and Sales et al. (28). Electron microscopic evidence for a synaptic location of PrPC has proven very difficult, however. Thus, it was necessary to use embedding techniques leading to destruction of cell membranes. As a consequence, the electron microscopic evidence for PrPC location in synaptic vesicles has been disputed. Biochemical studies showed that the prion protein is located predominantly in the synaptic plasma membrane (23) and, to a lesser extent, in the synaptic vesicle fraction. Fig. 2 shows a Western blot analysis of PrPC expression in various synaptic fractions. The enrichment of PrPC in the synaptic plasma membrane fraction is evident (Fig. 2A, lane 4).
Electrophysiological Studies
Electrophysiological studies in Prnp0/0 mice have been used to identify the function of PrPC in neurons. Collinge et al. (29) were the first to describe a change in long-term potentiation (LTP), i.e., a change of synaptic transmission after repetitive stimulation in the Prnp0/0 mouse generated by Bueler et al. (10). This finding was confirmed in a second Prnp0/0 mouse generated by Manson et al. (30). However, Lledo et al. (31) did not observe LTP changes.
In addition, Collinge et al. (29) found altered kinetics of the inhibitory postsynaptic currents (IPSCs), i.e., a prolongation of the rise time of GABAA receptor-mediated IPSCs in hippocampal neurons of Prnp0/0 mice. The authors argue that this may be caused by changes in the GABAA receptor on the postsynaptic membrane since a decrease of the amplitude of stimulated inhibitory postsynaptic currents and a shift of the reverse potential of GABAA receptor-mediated chloride currents were also observed.
Fig. 1. Synaptic expression pattern of PrPC in PrPC-overexpressing transgenic mice. Laser scanning confocal images of PrPc expression in the retina and cerebellar cortex of PrPC-overexpressing mice. Expression of PrPC (A) and synaptophysin (B) in Tg20 retina. PrPC is strongly expressed in the inner and outer plexiform layer, similar to synaptophysin. PrPC expression in Tg35 (C) and Tg20 (D) cerebellar cortex. Strong PrPC expression was observed in the molecular and granule cell layers in both transgenic mouse lines. However PrPC expression in Purkinje cells was only observed in Tg35 (C).
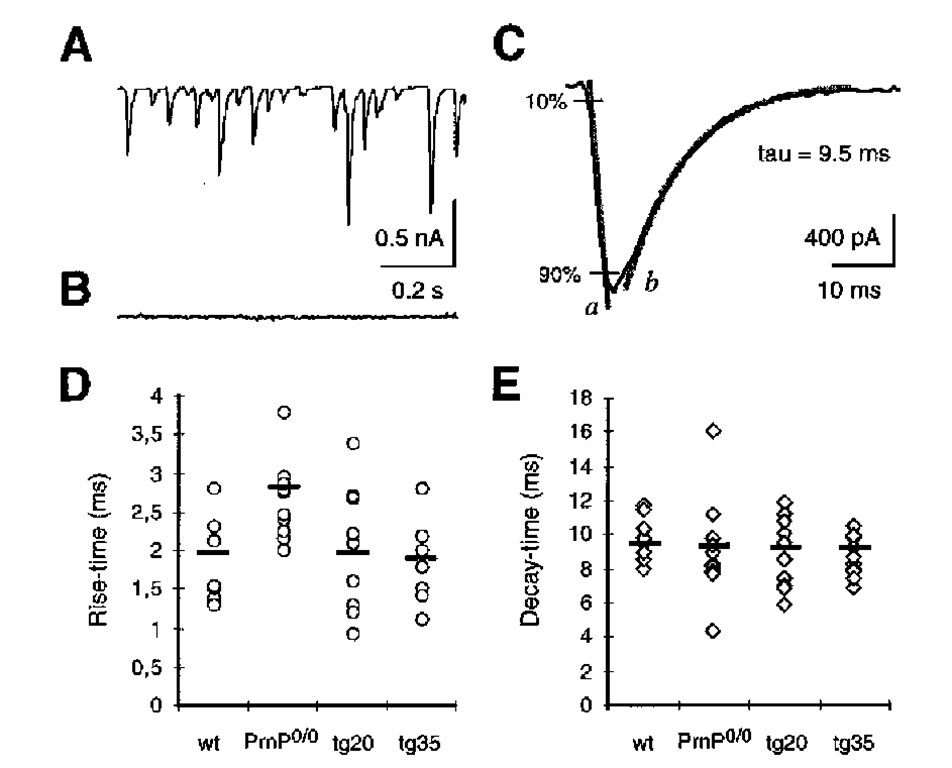
Lledo et al. (31) did not confirm this finding for hippocampal neurons of the same knockout line. Also, a more detailed analysis of the kinetics of GABAA-induced currents in outside-out patches from cerebellar Purkinje cells of Prnp0/0 mice did not reveal significant deviations from control cells (32). Moreover, studies on the kinetics of spontaneous inhibitory postsynaptic currents (sIPSCs) in cerebellar Purkinje cells of Prnp0/0 mice initially showed significant differences between the rise time of wild-type and that of Prnp0/0 Purkinje cells (32). Further experiments with Purkinje cells of younger animals, with a better voltage clamp (and consequently a more exact estimation of the rise time [33]) showed a significant increase in the rise time, from 1.9 ms in wild-type to 2.81 ms in Prnp0/0 mouse Purkinje cells (Fig. 3D; P = 0.001). No differences were found in the decay time (Fig. 3E). Evidence for the hypothesis that the increased rise time is caused by loss of the PrPC was found in studies on the rise time in Prnp0/0 mice that were Prnp reconstituted (Fig. 3D; Tg35; [34]). The IPSC rise time in Purkinje cells of these animals corresponds to the rise time in wildtype animals.
Fig. 2. Enrichment of PrPC in the synaptic plasma membrane fraction. Preparations of the synaptic plasma membrane fraction and synaptic vesicle fractions from synap-tosomes (54). Equal amounts (100 ^g/per lane) of brain homogenate and various subcellular fractions from wild-type (lane 1-4), Prnp0/0 (lane 6), and Tg35 (lane 7) mice were investigated in Western blots. The monoclonal antibody 3B5 (A); hybridoma supernatant 1:50) (55) was used to identify PrPC. A polyclonal antiserum (1:2000) was used to identify the synaptic vesicle protein synaptotagmin (B) (56). The N-methyl-D-aspartate (NMDA) receptor subunit, R1, was shown using the monoclonal antibody, Akp (C); (1:2000) (55,57). Subcellular fractions are designated as follows: lane 1, WT homogenate; lane 2, WT crude synaptic vesicle fraction; lane 3, WT cytosolic synaptic fraction; lane 4, WT synaptic plasma membrane fraction; lane 5, mol w. standards; lane 5 synaptic plasma membrane fraction from Prnp0/0 mouse brains. An enrichment of PrPC (A) is noted in the synaptic plasma membrane fraction of wild-type mouse (lane 4), in analogy to the subunit R1 of the NMDA receptor in lane 4 (C). In contrast to synaptotagmin, a protein that is predominantly localized to the membranes of synaptic vesicles, PrPC is not enriched in the synaptic vesicle fraction (lane 2), although it may be found in this location in low concentration.
To clarify the question of whether the increase in rise time in Prnp0/0 mice is caused by the loss of PrPC expression in the presynapse or postsynapse, an additional Tg line, which expresses PrPC only at the presynapse (Tg20) (34) was examined. In this line, rise times corresponding to the wildtype were found (Fig. 3D). Thus, it appears that the loss of the presynaptic PrPC expression at the inhibitory synapse is responsible for the prolongation of the rise time of inhibitory postsynaptic currents in Prnp0/0 mice.
Independent of the findings at inhibitory synapses, Colling et al. (35) described an additional electrophysiological phenotype in Prnp0/0 mice, i. e. a disturbance of the late afterhyperpolarization current, IAHP. This current is involved in action potential repolarization and therefore influences the frequency of action potentials. Colling et al. (35) reasoned that the disturbed IAHP in Prnp0/0 mice is caused by a decreased conductance of calcium-activated potassium channels, which may be related to a disturbed intracellular calcium homeostasis.
Fig. 3. Presynaptic PrPC expression modulates the kinetics of inhibitory postsynaptic currents (IPSC). (A), Spontaneous IPSCs from a Purkinje cell of a 10-d-old wild-type mouse using the patch-clamp technique, as described (32) (B), Using the effect of 10 ^M bicucullin, a y-aminobutyric acid A (GABAA) receptor blocker, it is shown that the synaptic currents are inhibitory GABAA receptor-mediated conductances. (C), rise time and decay time in wildtype IPSCs. During rise time, there is a linear increase of GABAA receptor-mediated current from 10 to 90% of the maximum (gray line a). The decay time (t) is calculated from the kinetics of an exponential function (gray line b) that shows the best fit to the actual decay of the current. (D), Rise time in WT, Prnp0/0, Tg20, and Tg35. Shown is the mean of results from each of 10 measurements in Purkinje cells of 9-12d-old animals. Each point corresponds to the rise time of inhibitory postsynaptic currents of a Purkinje cell (mean of the rise time of 20 consecutive IPSCs for each cell). The mean of all measurements is shown as black line. The IPSC rise time is significantly prolonged in Prnp0/0 mice compared to wild-type mice (p = 0.001, t-test according to Welch). No significant differences were found among the rise times of wild-type, Tg20, and Tg35 cells. (E), Means of the decay time of IPSCs in wildtype, Prnp0/0, Tg20 and Tg35. There are no differences among these mouse lines.
This concept is based on findings by Whatley et al. (36) that indicated an effect of recombinant PrPC on the intracellular calcium concentration in synaptosomes.
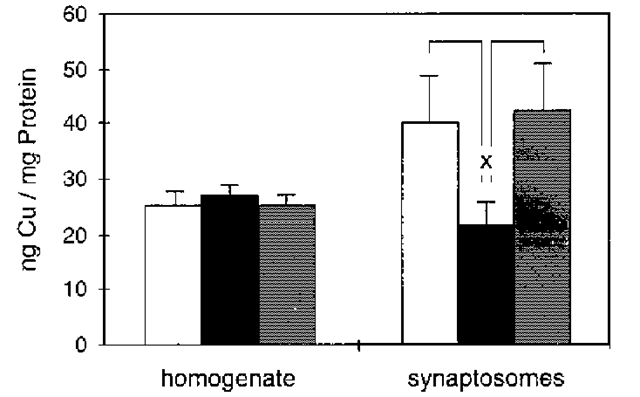
Fig. 4. Copper concentration in synaptosomes correlates with PrPC expression. The copper concentrations in whole-brain homogenates and synaptosomal fractions from wild-type (open columns), Prnp0/0 (black columns), and Tg20 (gray columns) mice were studied by atomic absorption spectroscopy. Shown are the mean and SE of the arithmetic mean of 3-7 preparations from each of five brains of age-matched (2 ± 0.4 mo) female animals of various lines. The copper concentration related to protein concentration in whole-brain homogenates shows no significant differences among wild-type, Prnp0/0 and Tg20 mice, but the synaptosomal fraction shows a significant reduction of copper in Prnp0/0 mice compared to wildtype and Tg20 mice (p = 0.03; t-test).
Indeed, a study of calcium-activated potassium currents in Purkinje cells of Prnp0/0 mice showed a reduced amplitude of these currents (Herms et al., in preparation). Further investigations of transgenic animals which were Prnp reconstituted on the Prnp0/0 background (Tg35, Tg20) showed that loss of PrPC expression in Purkinje cells is responsible for this finding (37). Thus, a reconstitution of the amplitude of calcium-activated potassium conductances was observed in a transgenic line that shows overexpression of PrPC in all neurons (Tg35), whereas a transgenic line that overexpresses PrPC in all neurons but Purkinje cells, showed no reconstitution of the amplitude. The subsequent microfluorometric investigation of the intracellular calcium homeostasis in Prnp0/0 mice confirmed that the reduction of calcium-activated potassium currents is probably caused by reduced calcium release from intracellular calcium-sensitive calcium stores (37) (Herms et al., in preparation).
The Role of Copper
The cause of the observed electrophysiological alterations in Prnp0/0 mice is not yet known. They may be related to the decreased copper concentration in synaptic membranes of Prnp0/0 mice (Fig. 4; [23]). The N-terminus of PrPC has a highly conserved octapeptide repeat sequence (PHGGGWGQ) x4 (38), whose possible copper-binding properties were first shown by Hornshaw et al. (39,40) and later by Miura et al. (41). The recombinant N-terminus of PrPC from amino acid 23 to 98 (PrP 23-98) shows a cooperative binding of 5-6 copper ions (42). Half-maximal cooperative copper binding of PrP23-98 is in the micromolar range (5.9 ^M). Further investigations, using synthetic octapeptides (43) confirmed cooperative copper binding by PrPC.
The significant decrease of synaptosomal copper concentration in Prnp0/0 mouse synaptosomes (Fig. 4) may be caused by a decreased reuptake of copper released into the synaptic cleft during synaptic vesicle release, since the difference in the synaptosomal copper concentration between Prnp0/0 mice and wildtype mice seems to be too large to be explained solely by the loss of copper bound to PrPC. In addition, one would then also expect differences in the copper concentration of the crude homogenate in wildtype, Tg20 and Prnp0/0 mice (Fig. 4). The findings may therefore be explained by a dysregulation of the copper concentration in the brains of Prnp0/0 mice caused by loss of PrPC.
In addition to the decreased synaptosomal copper concentration, a number of further changes were observed that indicated a biological function of copper binding by PrPC. Thus, significant differences between Prnp0/0 mice and wildtype mice were found in inhibitory synaptic transmission in the presence of copper (42). The application of copper elicited a significant reduction of the mean amplitude of spontaneous inhibitory postsynaptic GABAA receptor-mediated currents in Purkinje cells of Prnp0/0 mice at a concentration of 2 ^M Cu2+, whereas this concentration showed no effect on the IPSCs of the wildtype mice. Because it is well known that the GABAA receptor is functionally disturbed at a concentration of copper in the range of 1 ^M (44), this finding indicates that differences between Prnp0/0 and wildtype mice may be caused by missing copper buffering in the synaptic cleft by PrPC.
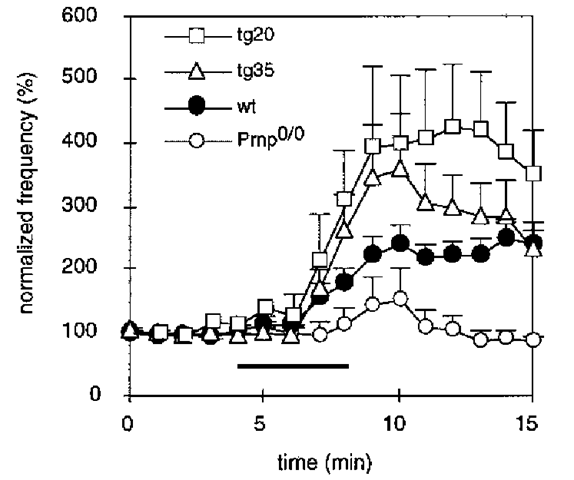
It is difficult to verify whether the loss of PrPC indeed leads to a reduction of the amount of copper located at the synaptic plasma membrane in intact synapses because direct synaptic measurements in vivo are not possible at present. We used an indirect approach to assess the problem of copper binding at the synapse, by studying the effect of hydrogen peroxide on inhibitory synaptic transmission (23). H2O2 is known to alter the probability of synaptic vesicle release by reacting with metal ions, particularly iron and copper at the presynapse, by increasing the presynaptic calcium concentration. By performing patch-clamp measurements on cerebellar slice preparations of wildtype, Prnp0/0 and PrPC reconstituted transgenic mice, we observed the effect of 0.01% H2O2 on the frequency of spontaneous IPSCs in Purkinje cells correlate with the amount of PrPC expressed in the presynaptic neuron (Fig. 5). This indicates that the amount of copper at the synapse may indeed be PrPC-related.
Fig. 5. Enhancement of inhibitory synaptic activity by hydrogen peroxide is related to the amount of PrPC at the presynaptic plasma membrane. Effect of 0.01% H2O2 on the frequency of inhibitory postsynaptic currents in the different mouse lines. Each point represents the mean ± SEM sIPSC frequency in 1-min intervals normalized to the values before H2O2 application of wild-type (n = 14), Prnp0/0 (n = 21), Tg35 (n = 15) and Tg20 (n = 4) mouse Purkinje cells. The bar indicates the time during which H2O2 was applied. The application of H2O2 led to a marked enhancement of synaptic activity in wild-type mice, there is no comparable effect in Prnp0/0 mice. In transgenic mice that overexpress PrPC on a Prnp0/0 background in all neurons (Tg35), the sIPSC frequency increase after H2O2 application is rescued. Also, PrPC-reconstituted mice, which express PrPC in cerebellar interneurons, but not in Purkinje cells (Tg20), show a rescue, indicating that the presynaptic PrPC expression is important for the rescue of the H2O2 effect on IPSC frequency.
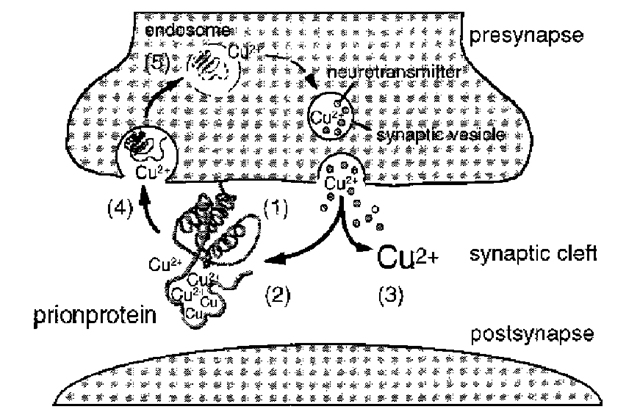
It remains to be shown whether buffering of copper released during synaptic vesicle release, which prevents or minimizes unspecific binding of copper to other proteins, is the primary function of PrPC (Fig. 6). Alternatively, the binding of copper to PrPC may primarily serve the reuptake of copper into the presynapse by endocytosis of PrPC (45,46) or may be of structural importance for the N-terminus of PrPC (47).
The hypothesis of a functional re-uptake of copper in the synaptic cleft by the prion protein (Fig. 6) may explain electrophysiological findings in Prnp0/0 mice, which, on first glance, seem contradictory. A slight increase of extracellular copper concentration, caused by decreased or missing copper buffering in the synaptic cleft in Prnp0/0 mice, may cause a decrease in the conductance of voltage-activated calcium channels and a change in the kinetics of the GABAA receptor.
Fig. 6. Hypothetical model showing a possible function of copper binding by PrPC at the synaptic plasma membrane. The prion protein is attached to the presynaptic plasma membrane (1) (23), where its N-terminal moiety (2) binds free copper that is released into the synaptic cleft with synaptic vesicle release (3) (58,59). There is an endocytotic uptake of PrPC into the presynapse (4) (45,46) where PrPC-bound copper is released, possibly induced by endosomal pH changes (5) (43). Thus PrPC serves to keep the copper concentration in the presynaptic cytosol and the synaptic cleft constant despite copper losses during synaptic vesicle release (3).
Thus, the conductance of the GABAA receptor and voltage-activated calcium channels, which modulate intracellular calcium homeostasis is clearly disturbed by copper concentrations of 1-10 ^M (44,48). This would explain the alteration of the intracellular calcium homeostasis in Prnp0/0 mice, changes in the conductance of calcium-related ion currents, and changes in GABAA receptor-related inhibitory postsynaptic currents observed under certain conditions. Reduced LTP in Prnp0/0 mice may be explained by this hypothesis, as well. As shown by Doreulee et al. (49), LTP is blocked by concentrations of free copper as low as 1 ^M. Changes in the circadian rhythm observed by Tobler et al. (12,13) in Prnp0/0 mice could be related to a disturbed copper uptake and a decreased activity of copper-dependent enzymes, since the synthesis of melatonin, which is important in the regulation of circadian rhythms (50), is regulated by the copper-dependent enzyme monamine oxidase (51). Also, the activity of two other copper-dependent enzymes, the Cu/Zn superoxide dismutase and the glutathione reductase have been found to be altered in PrP0/0 mice (52,53).
Conclusion
In summary, our studies have shown that PrPC binds copper cooperatively and with high affinity. In the brain highest concentrations of PrPC are found at synapses. Synaptosomes of Prnp0/0 mice demonstrate a strong reduction of copper concentration. Copper binding by PrPC in the synaptic cleft has a significant influence on synaptic transmission. It remains to be shown whether additional phenotypes observed in Prnp0/0 mice result from decreased copper binding or from a disturbance of copper distribution in the absence of PrPC.