2. The retroviral life cycle
The life cycles of simple and complex retroviruses are indicated in Fig. 3. Although both follow a similar pathway leading to establishment of the integrated provirus, they differ in subsequent events leading to maturation, where complex retroviruses have established control mechanisms for maintaining virion RNA within the nucleus. Salient feature of the life cycle include
• attachment to specific receptor(s) on the surface of the target cell
• penetrating and uncoating to release virion cores into the cytoplasm
• converting the single-stranded RNA genome into double-stranded proviral DNA
• transporting the "preintegrative" nucleoprotein intermediate into the nucleus
• integrating proviral DNA into the host genome
• synthesizing viral RNA from the integrated provirus
• transporting viral RNA to the cytoplasm
• synthesizing polyprotein precursors that encode structural and enzymatic proteins
• assembling nucleic acid and protein components at the cell membrane and budding
• maturing infectious virions
Figure 3. Life cycles of simple and complex retroviruses. For simple retroviruses, the life cycle can be envisioned as tw( discrete sets of events, (1) establishment of the integrated provirus and (2) expression of the provirus, followed by virion maturation. Although complex retroviruses establish the provirus similarly, then regulatory proteins (Tat, Rev, Rex) are synthesized from multiply spliced RNA (a) which aid in transporting unspliced and singly-spliced messages to the cytoplasm. Thereafter, (b) events follow the same pattern as simple retroviruses.
2.1. Attachment
The interaction of virions with a cell-surface receptor is mediated by the highly glycosylated SU component of the retroviral env gene. One of the most extensively characterized of these is the HIV receptor CD4, which functions in the host cell to aid in signaling the interaction of T-helper cells with antigen-presenting cells. Receptors closely resembling transport systems have been identified for both MLV (Rec-1 and Ram-1) and GALV (Glvr-1). Tva, the subgroup A ALSV receptor, is structurally distinct and bears some resemblance to the low-density lipoprotein receptor. Candidate receptors for BLV and FIV have been identified but require further characterization. Although the prevailing dogma envisages a single class of receptor-mediated attachment, the observation that murine model systems bearing the human CD4 receptor support attachment of HIV, but not the subsequent events, means that a second recepetor may be involved. This notion has recently been proven experimentally by the identification of chemokine receptors CXCR-4 (fusin) and CCR5 as important coreceptors in HIV infection (4). Although CXCR-4 mediates infection of CD4+ cells by T-cell line adapted HIV-1 strains, CCR5 allows entry of primary/macrophage tropic strains. Other macrophage-tropic and dual-tropic strains exploit the additional chemokine receptors CCR3 and CCR2b.
2.2. Fusion and Uncoating
Penetration and release of the virion nucleoprotein core occurs by one of two distinct mechanisms: (i) fusion between the viral envelope with the cell membrane or (ii) receptor-mediated endocytosis accompanied by fusion with the endosomal membrane. Such events in retroviruses are pH-independent and are mediated by hydrophobic amino acids at the amino terminus of the envelope transmembrane (TM) component. Studies with HIV provide compelling evidence that a cellular peptidyl-prolyl isomerase, cyclophilin A (CyPA), participates in events between membrane fusion and initiation of reverse transcription (5). CyPA is incorporated into virions late in the infection cycle through direct interaction with the CA component of gag precursor polyprotein, suggesting that it may participate directly in uncoating. The virion structure presented in Fig. 1 predicts that MA provides an outer shell and would not be associated with core particles after uncoating. However, this is not the case with HIV, where a small proportion of MA molecules remain core-associated, possibly to assist in transporting the preintegrative nucleoprotein complex into the nucleus.
2.3. Proviral DNA Synthesis
Proviral DNA synthesis occurs in virion cores whose structure is relaxed to allow access to deoxynucleoside triphosphates. Through the elegant series of events depicted in Fig. 4, positively-stranded viral RNA is converted into double-stranded proviral DNA by a single virus-coded enzyme, RT. However, because signals that determine initiation and termination of transcription by the host RNA polymerase reside within the integrated provirus, these by default are absent from the RNA genome of an invading virus. Therefore, a mechanism must be established for regenerating them in the course of the reverse transcription cycle.
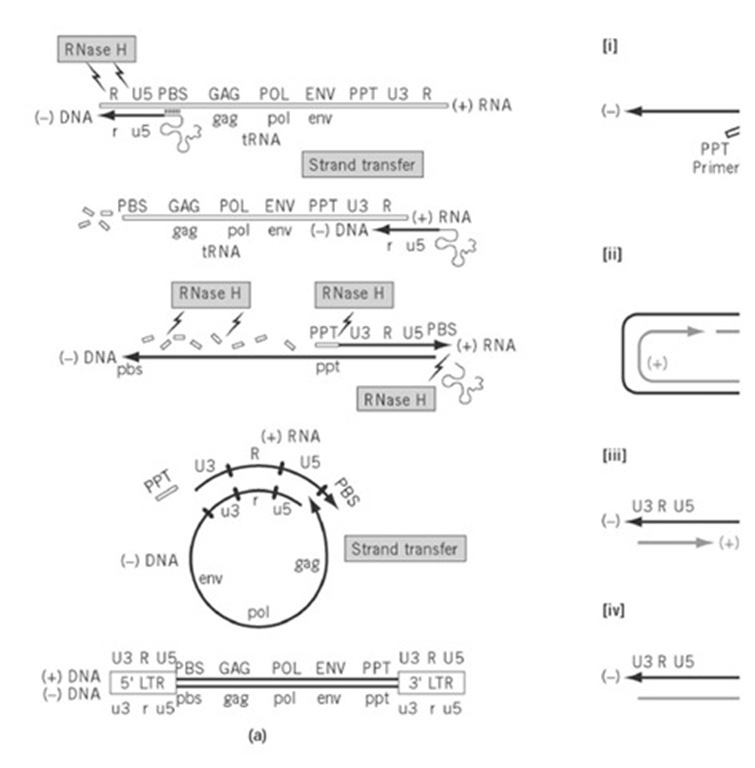
Figure 4. (a) Model for synthesizing proviral DNA (closed lines) from the single-stranded RNA genome (open lines). Fi text. The RNA genome that contains the coding information for protein synthesis is designated (+) RNA, from which a c Subsequent use of the (-) DNA template generates the (+) DNA strand. Between these events, the (+) RNA genome is d LTR elements are only partially represented at either terminus of the RNA genome and are regenerated following two DN mechanism for discontinuous (+) strand synthesis in lentiviruses and termination of 3′ PPT-initiated synthesis at the cent strands are represented by bold and gray lines, respectively. In Step (1), (+) strand synthesis initiates from both the 3′- ar PPT-initiated synthesis terminates within the tRNA template, and the tRNA is released. Step (2), second-strand transfer v homology of (-) and (+) DNA. Step (3), bidirectional strand displacement synthesis via elongation of the (-) strand and < of upstream (+) strand segment and its termination after CTS-controlled strand displacement at the center of the retroviral
First, minus-strand DNA synthesis is initiated from a host-derived tRNA that is hybridized via its 3′-terminal 18 nucleotides to the primer binding site (PBS) immediately downstream from U5. Examples of tRNA used to initiate replication include tRNATrp in RSV, tRNAPro in MLV, and tRNALys,3 in HIV. As U5 and R sequences are copied, RT-associated ribonuclease H (RNase H) activity degrades RNA of the RNA-DNA replication intermediate, thereby making R sequences of minus DNA accessible for hybridization to their complement at the 3′ end of the RNA genome. Through a process designated " strand transfer’ or "switching", nascent minus-strand DNA is relocated to the 3′ end of the same (intramolecular) or a different RNA genome (intermolecular). Continued RNA-dependent DNA synthesis creates a first copy of the LTR-U3-R-U5- hallmark on minus-strand DNA, and RNase H activity continues to degrade RNA of the replicative intermediate. The exception to this is a purine-rich RNA sequence located near the 3′ end of the RNA genome, designated the polypurine tract (PPT), which resists hydrolysis to serve as a primer for plus-strand DNA synthesis. Plus-strand, DNA-dependent DNA synthesis ensues, using newly synthesized minus-strand DNA as a template, up to and including the first 18 nucleotides of the tRNA replication primer (i.e., this is used as both primer and template during replication). At this step, the replication machinery transiently pauses at a methylated base of the tRNA, allowing RNase H-mediated removal of the intact minus-strand primer. As a consequence, plus-strand PBS sequences are made available for hybridization to their minus-strand counterparts to complete a second, intramolecular strand switch (concomitant with which the PPT primer is removed). Subsequent bidirectional DNA-dependent DNA synthesis generates a double-stranded proviral DNA flanked by complete copies of the LTR.
Although this model holds true for many retroviruses, several lentiviruses and yeast retrotransposons harbor a central plus-strand discontinuity, that reflects use of a second primer in the middle of the genome (the central or cPPT) (6). Although the advantage of an additional PPT is unclear, its alteration on the HIV genome has severe consequences for replication. The reverse transcription cycle that accounts for both 3′- and cPPT-initiated plus-strand synthesis is presented in Figure 4 (b). Documentation of a plus-strand discontinuity at the center of proviral DNA imposes an additional constraint on these retroviruses, namely, to provide a mechanism for terminating plus-strand synthesis immediately downstream from the cPPT. Preliminary evidence for this has been provided in HIV, where localized distortion of double-helical DNA geometry ("bending"), causes pausing and dissociation of the replication machinery. The DNA sequence and structure implicated in such events has been designated the central termination sequence (CTS). Finally, it should be pointed out that both copies of RNA in the virion core are used to synthesize a single proviral DNA, which indicates that relocation of nascent DNA between genome templates occurs frequently during replication. Because the retroviral polymerase is inherently more error-prone than host DNA polymerases, such recombination has the potential to enhance the rate of evolution, in addition to allowing RT to bypass lesions in the RNA genome.
2.4. Transport of Proviral DNA
Following DNA synthesis, the nucleoprotein complex composed of linear DNA, the core protein CA, RT, and possibly NC, is transported through the nuclear membrane (see Nuclear import, export). In some cases (eg, MLV), breakdown of the nuclear membrane at cell division is necessary to support these events, evidenced by the observation that inhibiting the onset of mitosis delays proviral DNA integration. An alternative strategy is employed by lentiviruses, which do not require the infected cell to enter mitosis. Nonenzymatic HIV-1 proteins, such as MA-derived peptides and Vpr, have been detected as constituents of the nucleoprotein complex, from which it has been postulated that nuclear localization signals of these accessory proteins assume a supportive role in transport (7).
Within the nucleus of infected cells, two variants of circular proviral DNA have been detected that contain either one or two copies of the LTR. Originally proposed as integration intermediates, it has been unequivocally demonstrated that these represent the products of aberrant reverse transcription that have no biological function.
2.5. Integration
Integration is a mechanism whereby proviral DNA is stably inserted into the host genome colinearly with the way it is synthesized by RT. This process is catalyzed by the integrase (IN) component of the pol open reading frame and is independent of host factors and external energy sources (8). The immediate LTR termini contain short, imperfect, repeat sequences (att sites) that as shown by mutagenesis studies, constitute essential cis-acting sequences recognized by IN. In the absence of an exhaustive analysis, there is insufficient evidence to suggest preferential sites of integration in the host genome. During integration (the catalytic mechanism for which is described later), proviral DNA sequences are characterized by loss of two nucleotides at either end, whereas target sequences immediately adjacent to the integrated proviruses contain a short (4 to 6 bp) duplication, whose size is characteristic of a particular retrovirus. Examples of this are a 5-bp duplication in HIV and a 6-bp duplication in ALSV.
2.6. Viral RNA Synthesis
In essence, integration completes the first half of the retroviral life cycle, after which viral DNA is preserved in the germ line and transcribed from the LTR promoter by host-coded RNA polymerase II. Duplication of terminal sequences in the provirus implies that transcriptional signals of equal strength reside in both the 5′- and 3′-LTR. In fact, only the former is productively used, suggesting that regulatory signals downstream of the 5′-LTR are involved. The full-length genomic transcript initiates at the upstream U3/R junction with a "5′-capped" guanosine nucleoside and is polyadenylated immediately after the downstream R/U5 junction. In addition to LTR-derived RNA, spumaviruses initiate RNA synthesis from a site upstream of the bel-1 gene, and additional transcriptional initiation sites have also been identified for MMTV. A later section deals with host-coded factors active on the LTR promoter and the role of the transactivators in augmenting gene expression.
2.7. Splicing and Transport of Viral RNA
In all retroviruses, the intact transcript is both the RNA genome and messenger RNA for synthesizing gag and gag-pol precursor polyproteins, whereas a singly spliced subgenomic transcript encodes the env precursor. Different mechanisms have evolved to maintain a balance between unspliced and spliced mRNA.
Simple retroviruses exploit a cis-acting sequence, designated the negative regulator of splicing (NRS), which interacts with components of the cellular machinery to reduce splicing efficiency. In contrast, to maintain this balance, complex retroviruses have evolved more elaborate systems, involving the accessory proteins Rex (HTLV) and Rev (HIV, Fig. 2), which are derived via multiple splicing shortly after integration (9). These accessory proteins interact with signals located within either the viral env mRNA (Rev) or the R/U3 region of 3′ LTR RNA (RxRE), which otherwise provide a barrier to the export of transcripts harboring such sequences that are characterized by complex secondary structures and designated Rex- or Rev-responsive element (RxRE and RRE, respectively). Additional interactions of this RNA-protein complex with components of the cellular transport machinery facilitate transfer of intact and singly spliced mRNA to the cytoplasm. As outlined in Fig. 3, low-level synthesis of transactivator proteins (Tat, Rev, Rex), shortly after infection or after transcription is activated, has the consequence that unspliced and singly spliced RNA are maintained in the nucleus. However, a threshold level of Rev or Rex is eventually achieved, so that intact and singly spliced RNA are efficiently exported to the cytoplasm and made available to the translational machinery for events leading to virion assembly. Although such elaborate mechanisms are absent in simple retroviruses, RNA containing introns (ie, unspliced) must likewise be transported to the cytoplasm, implying an alternative mechanism. Preliminary data from D-type MPMV and RSV suggest that this is facilitated by the interaction of a viral RNA sequence, designated the constitutive transport element (CTE), with host-coded factors.
2.8. Protein Synthesis
Spliced mRNA provide the template for synthesizing TM and SU components of the viral envelope (in this case on rough endoplasmic reticulum-associated polyribosomes), and accessory proteins and oncogenes, whereas intact, unspliced RNA fulfills two roles. A portion of these become genomes, and another directs synthesis of virion structural (gag) and enzymatic proteins (pol). The mechanism by which gag and pol components are synthesized is a good example of genetic "streamlining" in retroviruses, where different mechanisms have evolved to allow high-level synthesis of structural proteins and low-level synthesis of virion enzymes from the same mRNA (10). MLV achieves this via transitional suppression, a mechanism more often associated with prokaryotic organisms. A UAG termination codon is located between the gag and pol open reading frames, and to the larger extent terminates synthesis of the gag precursor. In about 5% of cases, however, the host translational machinery inserts a glutamine residue at this position, thereby overriding termination to synthesize a gag-pol precursor polyprotein. Because the gag and pol genes in most other retroviruses are not in the same reading frame, an alternative method must be employed. This involves ribosomal frameshifting, where slippage of the translating ribosome allows reading the base before (-1 frameshift) or after (+1 frameshift) together with the gag stop codon, thereby creating the triplet for an amino acid. A single frameshift event is required to cotranslate the gag andpol genes in viruses, such as ALV and HIV, whereas two frameshift events are necessary when the PR open reading frame is out of frame with both of these (eg, MMTV, HTLV, and MPMV). In a frequently recurring theme, spumaviruses, the exception to these models of pol gene synthesis, have evolved an independently spliced mRNA for the pol gene that has its own translational initiation codon (11).
2.9. Assembly and Budding
Following their synthesis, gag, pol, and env gene products, together with a dimeric RNA genome, assemble in an ordered fashion at the cytoplasmic face of the cell membrane, gradually becoming enveloped in forming an immature virion. Membrane targeting of gag and gag-pol precursors is aided by N-terminal fatty acid modifications of MA that locate this protein to its ultimate fate as the inner shell of the virion. Lack of myristylation on gag precursors of ALSV and some lentiviruses suggests participation of additional, as yet unidentified, components in membrane targeting. An interaction involving a cis-acting element located at the 5′ end of viral RNA (the encapsidation or Y sequence) and gag-coded NC is critical for packaging the genome. Based the general nucleic acid binding properties of NC and the amount present in virions, it is most likely that this protein interacts with and "coats" the RNA genome. CA, located between MA and NC, is appropriately positioned to provide the virion core shell after proteolytic maturation. Gag-pol precursors are most likely oriented at the membrane similarly. They often serve an additional role of sequestering (via the pol-coded RT) the appropriate host tRNA isoacceptor required to prime reverse transcription events after subsequent infection. Alternatively, complementarity between viral PBS sequences and the 3′ terminus of the cognate replication primer may achieve the same goal. Only low-level DNA synthesis activity has been reported for RT embedded within the ~170kDa gag-pol precursor, which may reflect a control mechanism preventing premature reverse transcription before virion budding. Spumaviruses are the exception to this. Virions contain significant amounts of proviral DNA, which may lie in the observation that the pol gene is expressed independently of gag.
2.10. Virion Maturation
Immature cores that contain the appropriate protein and nucleic acid components gradually bud from the cell, together with a portion of the plasma membrane concentrated with the Env gene products. Concurrent with or after budding, the virally-coded protease is activated and is responsible for proteolytic maturation of gag and pol products. Maturation is are accompanied by significant morphological changes. The most notable of these is formation of an electron-dense virion core from "doughnut"-shaped precursor polyproteins that previously concentrated at the inner surface of the budding particle. At this stage, virions are considered capable of initiating a new cycle of infection by interacting with cell-surface receptors.