Retroviruses comprise a class of single-stranded RNA viruses whose genetic information is propagated through a double-stranded DNA intermediate integrated into the host chromosome. First documented in 1904 in the form of a nontumor-forming agent, equine infectious anemia virus and retroviruses of avian, mammalian, reptilian and piscine origin have now been documented (Table 1). They are associated with a variety of neurological disorders, wasting diseases, immunodeficiencies, and malignancies. Clinically significant retroviruses include type 1 and type 2 human immunodeficiency virus (HIV-1 , HIV-2), the causative agents of acquired immunodeficiency syndrome (AIDS), and type 1 and 2 human T-cell leukemia virus (HTLV-1 , HTLV-2), which manifest themselves in adult T-cell leukemia (ATL), tropical spastic paraparesis (TSP) and HTLV-associated myelopathy (HAM). Despite the increasing public health problem posed by HIV and HTLV infection, retroviruses have also made a fundamental contribution to biotechnology and molecular medicine. Following the seminal discovery in 1970 that reverse transcriptase (RT) catalyzes the flow of genetic information from RNA into DNA (1, 2), this enzyme for cDNA synthesis has found widespread use in producing medically important recombinant proteins, such as cytokines, growth factors, and hormones. Furthermore, since 1989, molecularly engineered retroviral vectors are increasingly used as delivery vehicles for gene therapy. A recent and ambitious application of retroviral vectors involves the proposal to introduce attenuated strains of HIV as live viral vaccines to control the spread of HIV infection and AIDS.
Table 1. Retroviral Genera
|
Retrovirus Abbreviation |
Full Name |
Genus |
|
MMTV |
Mouse mammary tumor virus |
B-type |
|
ASLV |
Avian sarcoma-leukosis virus |
Avian C-type |
|
ALV |
Avian leukosis virus |
Avian C-type |
|
RSV |
Rous sarcoma virus |
Avian C-type |
|
FeLV |
Feline leukemia virus |
Mammalian C- |
|
type |
||
|
galv |
Gibbon ape leukemia virus |
Mammalian C- |
|
type |
||
|
MLV |
Murine leukemia virus |
Mammalian C- |
|
type |
||
|
REV |
Reticuloendotheliosis virus |
Mammalian C- |
|
type |
||
|
SNV |
Spleen necrosis virus |
Mammalian C- |
|
type |
||
|
MPMV |
Mason-Pfizer monkey virus |
D-type |
|
SMRV |
Squirrel monkey retrovirus |
D-type |
|
BLV |
Bovine leukemia virus |
HTLV-BLV |
|
HTLV |
Human T-cell leukemia virus |
HTLV-BLV |
|
BIV |
Bovine immunodeficiency virus |
Lentivirus |
|
CAEV |
Caprine arthritis-encephalitis |
Lentivirus |
|
virus |
||
|
EIAV |
Equine infectious anemia virus |
Lentivirus |
|
FIV |
Feline immunodeficiency virus |
Lentivirus |
|
HIV |
Human immunodeficiency virus |
Lentivirus |
|
SIV |
Simian immunodeficiency virus |
Lentivirus |
|
HSRV |
Human spuma retrovirus |
Spumavirus |
|
SFV |
Simian foamy virus |
Spumavirus |
|
CSRV |
Corn snake retrovirus |
|
|
SnRV |
Snakehead fish retrovirus |
|
|
VRV |
Viper retrovirus |
Unclassified |
|
WDSV |
Walleye dermal sarcoma virus |
1. General features of retroviruses
1.1. Structure, Classification, and Genomic Organization
Despite significant differences in genetic complexity, host range, and interaction with their host, retroviruses share a common virion structure, a schematic version of which is illustrated in Fig. 1. Virions are typically 80 to 100 nm in diameter, contain an electron-dense core, and derive their lipid bilayer envelope from the host during budding. The virion surface is decorated with the envelope (env) gene products, comprised of a large surface glycoprotein (SU) (3) connected through disulfide bond linkages to a smaller transmembrane protein (TM). Matrix protein (MA), the amino-terminal product of the gag precursor polyprotein, is located between the virion envelope and capsid, and it possibly interacts with the TM subunit. Consistent with the notion of membrane association, the amino termini of MA proteins are often modified by the addition of a fatty acid, most commonly a myristoyl group. A second gag product, capsid protein (CA), forms the major internal structure of the virion, the core shell. Although the core shell assumes a highly ordered structure, the manner in which this is accomplished through packing of CA monomers remains to be established. An electron-dense virion core harbors two identical copies of the positive-stranded RNA genome, hybridized to a host-derived tRNA replication primer at the primer binding site (PBS) and likely coated over its entire length with the nucleocapsid protein (NC). Thepol-coded enzymes protease (PR), RT, and integrase (IN) constitute additional core-associated proteins. Immediately following virion entry, RT and IN are required to synthesize and integrate, respectively, the double-stranded DNA provirus into the host genome, and PR serves to process multi-domain gag and gag-pol precursor polypeptides during virion assembly at later stages in the retroviral life cycle. In addition to these "generic" core-associated proteins, virion cores of several lentiviruses contain a fourth pol-derived enzyme, deoxyuridine triphosphatase (dUTPase), whereas those of primate lentiviruses harbor the accessory virus-coded proteins Vpr and Vpx.
Figure 1. Schematic representation of a typical retrovirus virion, illustrating the disposition of structural and enzymatic proteins. Within the virion core, the dimeric RNA genome is most likely completely coated with the NC product of the gag gene. The two-letter abbreviation for retroviral proteins is indicated in parentheses.
Before nucleotide sequence data defining genomic organization, electron microscopy was used to distinguish retroviruses according to morphological features, leading to their designation as type A, B, C or D.
1. A particles are strictly intracellular nucleocapsid structures (containing a dimeric RNA genome, gag and gag-pol precursors), examples of which are the intracisternal A particles (IAP) of several rodent species and the immature form of mouse mammary tumor virus (MMTV). Fully formed immature cores of the B-type, D-type and spumaretroviruses are included in this group.
2. B particles are defined as the extracellularly enveloped form of MMTV. Immediately following budding, B particles mature to form condensed acentric nucleocapsid, which contrasts with the hollow appearance of the immature intracellular form. A prominent feature of B particles is decoration of their surface with the SU product of the env gene.
3. C particles comprise the simple avian and mammalian viruses, examples of which include Rous sarcoma virus (RSV) and Moloney murine leukemia virus (MOMLV). Such particles can be visualized as crescent-shaped patches on the cell membrane during budding, that is, fully formed intracellular structures are not generally detected. An electron-dense core morphology is also assumed by C particles immediately after budding, reflecting proteolytic processing of structural precursor polyproteins by the virally coded PR. Unlike their B-type counterparts, surface projections are barely visible on C-type viruses.
4. D particles are exemplified by Mason-Pfizer monkey virus (MPMV). These share many features of B-type viruses (including an intracellular nucleocapsid) but differ primarily in that their surface projections are less prominent.
More recently, seven genera of retroviruses have been defined, based on combining of nucleotide sequence homology and genomic complexity. Examples of these are provided in Table 1. Although B-type and D-type viruses remain unchanged, now those of C-type have been subdivided into avian and mammalian retroviruses. Both classes of C-type viruses contain "simple" genomes, that is, they encode only structural and enzymatic proteins from their gag, pol, and env open reading frames. Distinct from these are several groups of "complex" lentiviruses that encode as many as six accessory proteins on their genomes, in addition to gag, pol, and env products: 1. Lentiviruses are a diverse group of mammalian retroviruses responsible for neurological and immunological diseases but not directly implicated in malignancies. 2. HTLV-BLV genus includes a small number of complex retroviruses associated with B- and T- cell lymphomas, in addition to certain neurological disorders. 3. Spumaviruses (alternatively designated foamy viruses because infected cells display highly vacuolated or "foamy" syncitia) comprise a class of complex retroviruses of simian, human, and feline origin with which no disease has currently been associated. A hallmark of spumaviruses is the length of their genomes (>11,000 nucleotides). Additional features of their life cycle that distinguish spumaviruses as a unique class of retroviruses include (i) alternative splicing mechanisms for production of an independent mRNA encoding enzymatic proteins of the pol gene; (ii), the presence of significant amounts of double-stranded proviral DNA in the virion, and (iii) an unusual site of transport for the preintegrative nucleoprotein complex.
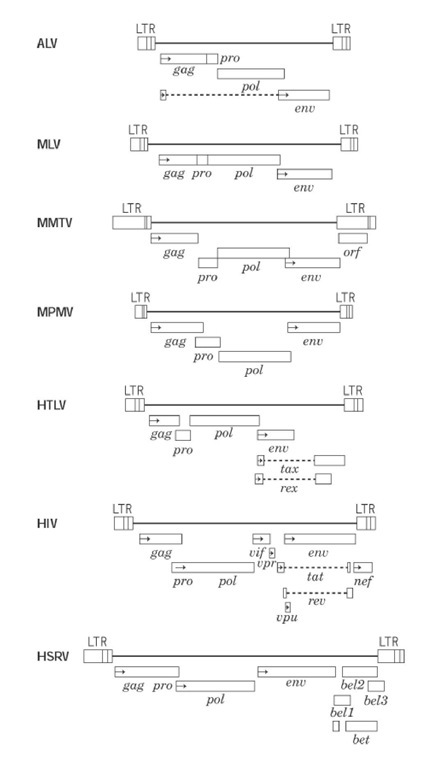
The organizations of several well-characterized retroviral genomes are indicated in Fig. 2. All proviral genomes are characterized by terminal noncoding regions (long-terminal repeats or LTR) that contain cis-acting functions necessary for replication, between which lie genes for proteins that have structural (gag and env open reading frames) and enzymatic roles (pol open reading frame). The same figure also highlights considerable differences in genomic complexity among different retrovirus genera. The simple -gag-pol-env- organization of ALV and MLV can be contrasted with HIV-1, which contains at least six accessory proteins derived via multiple RNA splicing events, the best characterized of which are the transactivators Tat and Rev. Through a complex series of interactions with highly ordered structures assumed by the viral RNA genome (the TAR loop with Tat and rev-responsive element or RRE with Rev), these proteins play important roles in controlling transcription of the viral genome and cellular localization of the RNA transcripts, respectively. Potential roles for additional HIV-1 proteins have included steps in virion maturation (Vif), nuclear localization of the viral preintegrative complex (Vpr), enhancement of virion release (Vpu) and CD4 receptor downregulation (NEF). Although controversy still surrounds the biological role(s) of these factors, several studies have shown that viruses within which Nef has been deleted or prematurely terminated are attenuated (i.e., less virulent), suggesting an important role for this protein in pathogenicity.
Figure 2. Coding regions of simple and complex retrovirus genomes. The provirus is presented in each case and is bracketed by LTR. Each box under this represents an open reading frame, within which horizontal arrows denote the initiation codon. Dotted lines designate coding regions joined by splicing events. The retroviral protease (pro) can exist as a component of the gag or pol open reading frames or in an independent reading frame.
Counterparts to HIV Tat and Rev proteins have been detected in most, if not all, lentiviruses. In the HTLV-BLV class the Tat counterpart, Tax, fulfills a similar role of transcriptional activation but through an alternative mechanism. Rather than interacting with a control element located in the RNA transcript, the target of Tax action is a segment of the retroviral promoter that contains three 21-bp repeat elements. Current evidence suggests that Tax does not interact directly with these motifs but rather with a member of the cyclic AMP response (CREB) family, which shares the same recognition element on the LTR promoter, thereby stimulating host transcriptional machinery. In contrast to Tax, HTLV Rex functions similar to the lentiviral proteins, in this case interacting with its RxRE target sequence on nascent viral RNA to control the levels of singly and multiply spliced transcripts in the cytoplasm. Of the several bel genes (between env and LTR) described for spumaviruses, the best characterized is the bell product, which functions analogously to HTLV Tax.