Whale lice (cvamids) are crustacean ectoparasites living on the skin of some species of cetaceans. Whale lice remain among some of the world’s biologically most specialized but least understood crustaceans. The current lack of knowledge about these animals undoubtedly stems from the fact that their natural habitat is limited to the skin surface of primarily slow-moving baleen whales, which themselves are difficult to study because they are generally submerged and constantly moving. Accordingly, it is very difficult for scientists to observe, in their natural setting and over extended periods of time, the behavior and biologic processes of whale lice. Some features have been learned, however, by a small number of scientists who have studied these interesting crustaceans for over a century, and the following brief account reviews these data.
I. Classification
Whale lice are actually not lice but perhaps they acquired this nickname in the 1800s from whalers who noticed that they crawled, presumably as parasites, on the surface of the whale’s skin and that their size, in proportion to that of a great whale, was comparable to the size of louse on a human or dog. Whale lice are arthropods of the subphylum Crustaca, class Malacos-traca, order Amphipoda, family Cyamidae, and genus Cyamus. As such they are closely related to other more commonly observed amphipods such as sand hoppers and caprellids, i.e., common marine peracarids. Altogether there are more than 5000 crustacean species, but only 23 known species of whale lice or cyamids. At least 15 of these comprise the genus Cya-mus. Because the practice of whaling has such a long history, cyamids were historically documented early, including Cyamus ceti by Linne in 1758, a genus described further by Latreille in 1796. A number of species were classified in the 1800s, including C. ovalis in 1834, C. delphini in 1836, C. boopis in 1870, and C. scammoni in 1872, and these cyamids were found on southern right whales (Eubalaena australis), humpback (Megaptera novaeangliae), gray (Eschrichtius robustus), and other large whales. Species names have occasionally been revised for cyamids and new species were discovered even late in the 20th century, such as the two new cyamids reported on one of the large beaked whales, the Baird’s beaked whale Berardius bairdii (Waller, 1989). Genus names for cyamids have also been changed over time and the earlier genus Paracyamus is no longer considered valid.
II. General Ecology and Morphology of Cyamids
Although information does not exist on the early development of cyamids, their evolution has undoubtedly been closely coupled with the long evolution of cetaceans, which first began around 55 million years ago when terrestrial species returned to the marine environment as precursors of the modern whales. Thus, modern cyamids show little resemblance to other crustaceans and have become greatly specialized for their parasitic and lifelong relationship to whales. They have a high degree of host specificity to whales, and one species, Cyamus boopis, is found only on humpback whales and another species, C. scammoni, only occurs on the gray whale. Some other species of whale lice overlap their residence on two to four species of whales. The two isolated Arctic odontocetes, beluga whales (Delphinapterus leucas) and narwhals (Monodon monoceros).
Each share the same two cyamids, C. monodontis and C. no-dosns. Table 1 summarizes the cetacean distribution of cyamids. Cyamids are unable to swim freely in the sea or from whale to whale at any of their developmental stages. Accordingly, they die if they lose their foothold on their host, but can be transferred from cetacean mother to calf or during cetacean mating. Although details on the number of juvenile stages that occur prior to adulthood are not yet established for cyamids, Leung (1976) has estimated that at least seven to eight instar stages exist for C. scammoni of the gray whale.
Although the general gross body structure of cyamids has been described elsewhere (Margolis, 1955; Leung, 1967; Berzin and Vlasova, 1982), very few studies have been directed toward the microscopic anatomy of cyamids. Early work on the musculature and very recent work (Levin and Pfeiffer, 1999) on cyamid ocular structure have been reported. Their small, paired eyes appear almost rudimentary. However, ultrastructural analysis of these photoreceptors for C. ceti has revealed well-developed sensory organs with each eye containing about 50 visual ommatidial units and an overall organization similar to other amphipod compound eyes. The exoskeleton of cyamids consists of a chitinous cuticle that is similar to that typically observed for other crustaceans. It has an exocuticle with multiple microfibrillar lamellae and an endocuticle traversed by both pore canals and dense fibers, as revealed by electron microscopy (Pfeiffer and Viers. 1998).
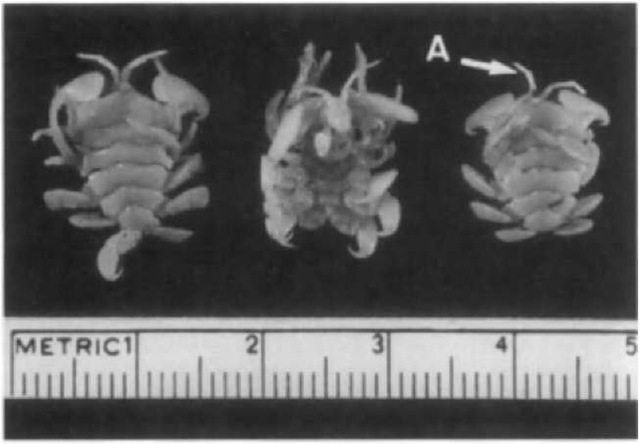
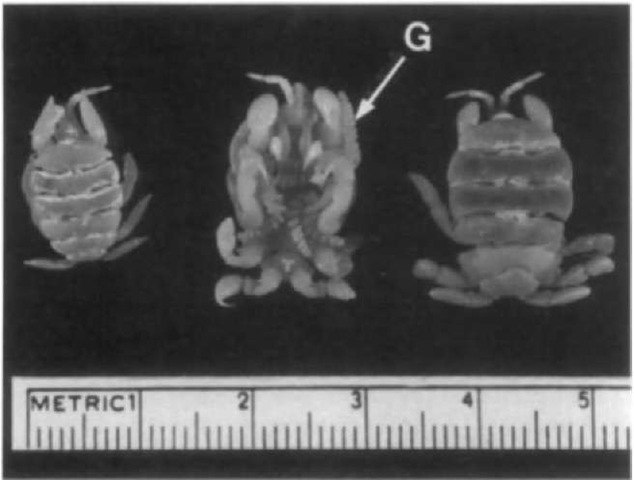
Cyamids are sexually dimorphic. The males are larger and, depending on species, adult cyamids usually range from approximately 6 to 19 mm in length. The most striking features of their appearance are their marked degree of segmentation and prominent gnathopods, or legs, with large dactyli, or hooks, that assure firm attachment to the host. The body is flattened and divided into a small cephalic-cephalon or head with paired, minute eyes, and segmented pereion or body to which are attached two pairs of gills and four pairs of gnathopod-type appendages. Figures 1 and 2 illustrate examples of the general body structure for C. ovalis and C. scammoni, respectively.
Cyamids are mostly found on those areas of the whale surface most protected from the turbulence of water flow, which on baleen whales include regions around barnacles, skin folds or ventral grooves of the head, protected zones around the blowholes, eyes, and flippers, margins of the lips, on callosities, wounds, and genital slit. In those species of whales that serve as host to several species of cyamids, there may be differences in the spatial distributions of the different species of whale lice, and within one species of cyamid the reproductive status and sex of the cyamid may alter the spatial distribution (Rice and Wolman, 1971; Balbuena and Raga, 1991; Rowntree, 1996). Whale lice do move around on their cetacean hosts; in the case of C. boopis of the humpback whale, the larger males may carry their smaller female mates and, in an artificial aquarium setting, were observed to walk at a rate of 4.5 m/hr (Rowntree, 1996).
Whale lice breathe by means of two pairs of external gills, which are much reduced in size in early juvenile stages. It has been reported that they can live for up to 3 days out of an aquatic environment, such as on a stranded whale, suggesting that they can also rely on integumentary respiration (Leung, 1976).
TABLE I
Distribution of Cyamid Species on Cetaceans”
|
Host |
Whale lice |
|
Mysticeti |
|
|
Bowhead whale. Balaena mysticetus |
Cyamus ceti |
|
Right whales. Eubalaena spp. |
C. ceti, C. erraticus, C. gracilis, C. ovalis, C. catadontis |
|
Gray whale, Eschrichtius, robustus |
C. ceti, C. kcssleri, C. scammoni |
|
Blue whale, Balaenoptcra musculus |
C. balaenopterae, C. bahamondei |
|
Humpback whale, Megaptera novaeangliae |
C. boopis, C. elongatus |
|
Common minke whale, B. acutorostrata |
C. balaenopterae |
|
Fin whale, B. plnjsahis |
C. balaenopterae, C. bahamondei |
|
Odonticeti |
|
|
Sperm whale, Physeter macrocephalus |
C. ovalis, C. catadontis, C. bahamondei, Isocyamus delphini, Neocyamus |
|
physeteris |
|
|
Baird’s beaked whale, Benardius bairdii |
Platycyamus flaviscutatus, C. orubraedon |
|
Beluga, Delphinapteris leucas |
C. monodontis, C. nodosus |
|
Narwhal, Monodon monoceros |
C. monodontis, C. nodosus |
|
Northern and southern bottlenose whales, Hyperoodon spp. |
C. thompsoni, I. delphini |
|
Long-finned pilot whale, Globicephala melas |
Isocyamus delphini |
|
Short-finned pilot whale, G. macrorhynchus |
I. delphini |
|
Short-beaked common dolphin. Delphintis delphis |
I. delphini, syncyamus pseudorcae |
|
Risso’s dolphin, Grampus griscus |
I. delphini |
|
White-beaked dolphin, Lagenorhynchus albirostri-s |
I. delphini, Scutocyanms pai-vus |
|
Harbor porpoise, Phocoena phocoena |
I. delphini |
|
Killer whale, Orcinus orca |
I. delphini, C. antarcticensis |
|
False killer whale. Pseudorca crassidens |
I. delphini, S. pseudorcae |
|
Rough-toothed dolphin, Steno bredanensis |
1. delphini |
|
Gervais’ beaked whale, Mesoplodon densin<*tris |
I. delphini |
|
Pantropical spotted dolphin, Stenella attenuata |
Syncyamus sp. |
|
Striped dolphin, S. coeruleoalba |
Syncyamus sp. |
|
Spinner dolphin, S. longirostris |
Syncyamus sp. |
|
Common bottlenose dolphin, Tursiops truncatus |
Syncyamus sp. |
“From Margolis (1955). Leung (1967, 1970), Lincoln and Hurlev (1974), and Berzin and Vlasova (1982).
III. Feeding Habits of Cyamids
It was speculated for a long time that whale lice fed on whale skin and hence they were deemed ectoparasites. Some workers suggested that they might be ominvorous and ingest algal filaments or suspended materials or plankton in the water near their attachment site. However, their mouth parts are very small compared to their body size and they do not possess claws such as some other crustaceans or food-gathering cirri such as some predatory, sessile crustacean barnacles (Pfeiffer and Lowe, 1989). Cyainids have poorly developed paired mandibles and incisor processes with strong chitinous teeth that appear well suited for piercing and scraping skin. Rowntree (1983) showed that the color of intestinal contents of cyainids from humpback whales matched the skin color (black or white) from which the cyamids were collected. More recent conclusive evidence has proven that whale skin is a principal dietary material of cyamids. Both electron microscopic proof of whale skin keratinocytes within the upper digestive tract of cyamids and stable isotope evidence have shown that the dietary staple of whale lice is whale skin. Analysis of stable carbon and nitrogen isotope ratios from cyamids and skin from six species of whales have shown that the cyamic ratios closely matched those of whale skin, but not those of zooplankton from the sea where the cyamids and whales reside (Schell et al, 1999). Also supporting this conclusion is the evidence of direct damage to the skin by whale lice (Leung, 1976), Thus, cyamids have evolved into the only obligate parasites among the amphipods in distinction to other amphipods, such as caprellids, which are predatory and feed on diatoms, other crustaceans, and so on.
IV. Reproduction in Cyamids
Reproductive and mating behavior has been less studied in cyamids than in other amphipods and, indeed, has not been investigated in most cyamid species. The males practice mate guarding and consorts are formed, but there appears to be less aggressive territorialism than is evident with some other amphipods. Little is known about cyamid copulation. There is morphological evidence of a secretory product being released on the cuticular surface of amphipods (Pfeiffer and Viers, 1998), but it is not known if this serves as a pheromone-type attractant for mates or serves some other function. Electron microscopic evidence has shown many tactile sensillae on the antennae and head regions of cyamids, some of which are also likely chemoreceptors. One can question if they always sit on their sole food source, why they have evolved so many sensillae. Female cyamids have a brood pouch (four-plated) or marsupium on their ventral surface, and both unhatched eggs and juvenile whale lice are retained in this cavity. A clutch of 1078 eggs was observed in the marsupium of one female C. scammoni (Leung, 1976). The young cyamids measure only about 0.5 mm in length and crawl in and out of the marsupium during development and remain there for at least 2-3 months, when they become about 1.5 mm in length for C. scammoni. Several workers have proposed a seasonality for cyamid reproduction, but partly due to the migratory habits of whales, detailed data are not yet available on potential seasonal changes.
Figure 1 Three specimens of Cyamus ovalis showing ventral surface on center specimen. The head region faces the top. Note segmented body and antennae (A).
Figure 2 Three specimens of Cyamus scammoni showing ventral surface on center specimen. The gills (G) of this species have a spiral shape.