Individual identification is an important tool for studies of animal behavior, ecology, and population biology. Much • can be learned from recognition of individuals within a population or social unit, or from tracking individuals through time. Repeated observations of a recognizable individual can help to define its ranging patterns or site fidelity or to quantify habitat use. Behavioral studies benefit greatly from the ability to recognize individuals. Individual identification is essential to understanding group compositions, and this understanding is enhanced when the individual’s gender, age, genetic relationships, and reproductive condition are known. Similarly, interpretation of social interactions requires the ability to distinguish between the players. Behavioral descriptions often involve measurements of rates of occurrence of behaviors. These rates are measured most accurately when a selected individual is followed through time or when the individual’s behaviors are recorded at predetermined intervals, a process referred to as focal animal behavioral observations (Altmann, 1974).
Descriptions of life history patterns and empirical measures of population dynamics can be facilitated by individual identification (Hammond et al, 1990). By following individuals through time it is sometimes possible to determine age at sexual maturity, calving intervals, calf survivorship, and life span, providing measures of reproductive success. Combined, such individual measures can provide population level vital rates, including birth rates, mortality rates, and recruitment (e.g., Wells and Scott, 1990). Mark-recapture techniques use individual identification to arrive at abundance estimates. Individual identification provides one of the best tools for documenting exchanges of individuals between populations, allowing estimation of rates of immigration and emigration.
Selection of specific identification techniques depends on the research questions being addressed and the species under study. Frequent monitoring of individuals may require the ability to readily identify animals from a distance at each encounter, whereas other studies may only need to recognize an animal when it is handled subsequently, alive or at the end of its life. Some species exhibit individually specific natural markings that facilitate identification in the field. Other species lack such distinctive markings and require the attachment of artificial marks, or tags, if individual identifications are desired. Some species are visible on land at times, whereas others are entirely aquatic. Morphological, behavioral, and ecological features must be considered in order to determine what kind of tag or attachment is most appropriate in terms of safety to the animal and effectiveness for the research. It is now also possible to collect small samples of tissues that allow the identification of individuals genetically. Individual identification techniques have been summarized recently for cetaceans, pinnipeds, and sirenians (Hammond et al., 1990; Scott et al, 1990; Wiirsig and Jefferson, 1990; Erickson et al, 1993; Wells et al, 1999).
I. Cetaceans
A. Natural Markings
Cetaceans exhibit a variety of individually distinctive natural features. In most cases, features appearing above the surface of the water during the respiratory cycle are most useful. In particular, heads, backs, dorsal fins, and flukes are used most frequently for individual identification, with variations occurring in color patterns, skin patches, body scarring, and nicks and notches along fin edges (Hammond et al, 1990). Some individuals of most cetacean species acquire distinctive scars from previous wounds or injuries, which are often used for identifications. Perhaps the most unique features used to identify individual cetaceans are the callosities of the right whales, Eu-balaena spp. (Payne et al, 1983). These individually distinctive raised patches of roughened skin are present on the rostrum anterior to the blowholes in a pattern referred to as the bonnet, on the chin, lower lips, above the eyes, and near the blowholes. Whale lice, cyamid crustaceans that frequently live on the callosities, often give them a white, orange, yellow, or pink appearance. Callosities have allowed for the reliable recognition of individuals over periods of decades.
Color variations, where they exist among cetacean species, have been used with much success for individual identification, especially among the mysticetes (Hammond et al, 1990). Reminiscent of “Moby Dick,” a few anomalously white individuals have been noted for several species of large and small cetaceans, offering unusual opportunities for individual identification. Blue whales (Balaenoptera musculus) and gray whales (Eschiichtius robustus) exhibit individually distinctive mottling on their backs (Fig. 1). The dorsal fin and dorsal ridge, respectively, are used as reference points for locating mottling patterns on these species. Bowhead whales (Balaena imjsticetu.s) often have a distinctive pattern of white pigmentation on the chin and/or caudal peduncle. These patterns are readily seen from aircraft, the most commonly used observation platform for this arctic species. Fin whales (Balaenoptera plujsalus) exhibit strongly asymmetrical bodv pigmentation, with the lower and upper lips and first third of the baleen on the right side of the head appearing white or pale gray, while the left side lips and baleen are dark. A light-colored “blaze” sweeps back on the right side, and a V-shaped light-colored “chevron” occurs on both sides behind the blowhole. Minke whales (B. acutorostrata and B. bonaerensis) exhibit a pattern of pale lateral pigmentation on the body, often divided into three distinct swaths, with the relative brightness of the three swaths apparently varying consistently between northern and southern hemispheres. The distinctive dark and white patterns of the flippers and ventral surface of the flukes are familiar identification features lor humpback whales, Megaptera novaeangliae (Katona et al, 1979).
Some of the smaller cetaceans also exhibit useful color variations, from the perspective of the researcher. Most notable are the light colored saddled patches behind the dorsal fin of the killer whale (Orcinus orca), which differ in size and shape. Similar features are used for short-finned pilot whales (Globi-ccphala macrorhi/nchus), though the saddle marks are less distinct. Dorsal fin and/or back pigmentation variation has proven useful in studies of Dall’s porpoises (Phocoenoidcs dalli), Pacific white-sided dolphins (Lagenorhynchus obliquidens), Risso’s dolphins (Grampus griseus), and Hector’s dolphins (Cephalorlnjnchus hectori), and facial color patterns have been used to identify baiji (Lipotes vexillifer). Extensive speckling develops with age in spotted dolphins (Stenella attenuata and S. frontalis). Such speckling has provided much opportunity for individual identification from both above and below the waters surface in behavioral studies of Atlantic spotted dolphins.
A variation of the color pattern is scarring that results in pigment variations. For example, Risso’s dolphins acquire distinctive long-term white scars on their otherwise brown or gray bodies, and belugas (Delphinapterus leucas) acquire dark scars on their otherwise white bodies. Bottlenose dolphin (Tursiops spp.) scars on the dorsal fin often are white, in contrast to their general gray coloration. Cookie-cutter shark (Isistius spp.) bite wounds leave permanent small-diameter oval-shaped scars that are often depressed and pigmented differently from the rest of many pelagic cetaceans’ bodies.
Dorsal fins typically are prominent features that are visible to researchers during most cetacean surfacings. In many cetacean species, dorsal fins develop distinctive shapes or acquire nicks and notches, often through intraspecific or interspecific interactions, that allow for individual identification. Among the larger whales, fin, sei (Balaenoptera borealis), Bryde s, minke, humpback, and sperm whale (Physeter macro-cephalus) dorsal fins serve as useful identification features. Building on the pioneering work of Bigg (1982) with killer whales and Wiirsig and Wiirsig (1977) with bottlenose dolphins, studies based on dorsal fin identifications of various delphinids and other small cetaceans have blossomed in the last quarter century (Hammond et al, 1990; Scott et al, 1990; Wiirsig and Jefferson, 1990; Wells et al., 1999). Species that have received the most attention include killer whales, bottlenose dolphins, pilot whales (Globicephala spp.), humpbacked dolphins (Sotisa spp.), white-sided dolphins (Lagenorhynchus acutus and L. obliguidens), dusky dolphins (L. obscums), Risso’s dolphins, spinner dolphins (Stenella longirostris), Atlantic spotted dolphins, Heaviside’s dolphins (Cephalorhynchus heavisidii), Hectors dolphins, harbor porpoises (Phocoena phocoena), Amazon River dolphins (Into geoffrensis), tucuxi (So-taliaflunatilis), and baiji (Fig. 2). The frequency of occurrence of distinctive fin features varies from species to species, and in some cases from population to population. Along the central west coast of Florida, approximately 60-80% of bottlenose dolphins are considered to be distinctive based on dorsal fin features. Unlike color patterns that van1 from one side of the animal to the other, dorsal fin features are often equally visible from both sides and are distinctive under a broad range of lighting conditions, facilitating data collection in the field.
Figure 1 Distinctive color patterns of a blue whale.
Figure 2 Killer whale dorsal fins and saddle patches provide reliable identification cues.
Some cetacean species regularly lift their flukes from the water prior to a dive, providing predictable opportunities for researchers to note the occurrence of nicks, notches, and other features on the trailing edge of the flukes. Humpback whales offer both distinctive color patterns as well as trailing edge features for identification (Fig. 3). Humpback whale flukes were among the first natural markings on cetaceans to be recognized for their individual specificitv, and the technique has achieved extensive application worldwide in studies of population size and structure (Smith et al, 1999). Sperm whales also demonstrate much individual-specific variability in fluke edge features.
Figure 3 Distinctive dark and light patterns on the ventral surface of a humpback whale’s fluke.
Many of the cetacean features used by researchers for individual identification are visible above the surface of the water only briefly during respiratory cycles or are too subtle to be of use for accurate identification in real time. Most cetacean individual identification research involves the collection of permanent records of the distinctive features for subsequent detailed analvsis through a process generally referred to as photoidentification. As the name indicates, the process frequently involves 35-inm photography of cetaceans. Advances in digital imaging through still cameras and video are expanding the capabilities and possibilities for individual identification and facilitating image processing, storage, and sharing.
At its most basic level, photoidentification involves trying to obtain high-quality, high-resolution, full-frame images of identifying features (Wtirsig and Jefferson, 1990). Although photoidentification can sometimes be accomplished from shore, typically scientists in research vessels, whale-watching boats, or aircraft attempt to place themselves in position to be able to obtain an image of the features that is parallel to the photographic plane (lens oriented perpendicular to the feature of interest). Telephoto lenses aid the researcher in enlarging the features to fill the photographic frame. Motor drives or video allow multiple images to be taken in quick succession to optimize capturing fins, backs, or flukes at their greatest perpendicularity and height above the waters surface, for example. Data backs that print the time and date on the image provide additional assurance that images and data records can be matched correctly during subsequent analyses. Film selection varies with species, lighting conditions, and researcher preference. The film must have sufficiently fine grain to be able to resolve distinctive features, while allowing a shutter speed setting sufficiently fast to “freeze” the animal but slow enough to optimize depth of field for focus. Some researchers use black and white film, especially if expense or ease of manual processing is a concern. Color film is often used when documentation of wounds or the freshness of fin features is desired, for example. The use of high-resolution digital cameras is rapidly gaining acceptance and will eventually make conventional filming a thing of the past.
Techniques for image storage, retrieval, and analysis vary greatly across research situations. Often, images in the form of slides, prints, or negatives are labeled and stored chronologically in archival plastic sheets in binders and then examined under magnification through a hand-held loupe or dissecting microscope. In recent years, computer scanning of images into files suitable for electronic storage or transmission over the Internet has become increasingly popular. Such scanning also facilitates computer-assisted automated analysis. Previously, photographic matches were made through the laborious process of individual comparison by eye of the image of interest to all possible matches in a catalog of distinctive individuals. Computer software has been developed that can search thousands of images of such animals as sperm whales, humpback whales, or bottlenose dolphins in a very short time to produce a limited set of potential matches. The researcher can then make the final match using the exceptional resolving capabilities of the human eye. Additional rigor is often incorporated into the process through the use of multiple judges for difficult final identifications. Computer-assisted matching is becoming increasingly important as catalogs are now incorporating many thousands of individuals, and as contributions to centralized catalogs are being made by numerous researchers in widely dispersed locations.
Other kinds of “natural markings” that are being used increasingly are genetic markers from skin biopsy samples. Molecular analyses of small samples allow determination of gender and individual identification from genotypes provided by mi-crosatellite loci. This technique was developed for large-scale use during an ocean basin-wide study of humpback whales in which photographs were used to identify 2998 individual whales and microsatellite loci were used to identify 2015 whales (Smith et al, 1999). Based on the results of these initial studies, molecular techniques hold a great deal of promise for studies of a variety of cetaceans.
B. Temporary Markings
Natural temporary markings include skin lesions on parts of the body visible to researchers (Wilson et al, 1999) and soft-bodied barnacles that attach to dorsal fins, for example. Such markings can be useful for distinguishing between otherwise unmarked animals within a group, but their changeable nature make them less reliable for accurate identifications over long periods. Skin lesions may take weeks to months to fully heal and disappear, but their characteristics change during the healing process. Soft-bodied barnacles favor dorsal fin tips for attachment, leading to low variability in positioning, thus minimizing their value for identification.
Anthropogenic temporary markings have been found to be of limited utility with cetaceans (as reviewed by Scott et al, 1990). Remotely applied paint and tattoos have been tested with small cetaceans, and in all cases the animals were either not reidentified or the markings disappeared within 24 hr due to skin sloughing. In some cases, zinc oxide-based, brightly colored sun protection ointments have been applied to dolphins’ dorsal fins prior to release. These have allowed for the short-term identification of animals otherwise lacking in distinctive marks, and transfer of colors between animals can indicate social interactions.
C. Scarring and Branding
Dorsal fin notching has been attempted in a few cases with killer whales, bottlenose dolphins, pantropical spotted dolphins (Stenella attenuata), and spinner dolphins (Scott et al, 1990). Notching provides the same kinds of features used in the photographic identification of natural marks. Such notching requires capturing the animals, which also provide opportunities to learn the sex and age of the marked dolphin, as well as other biological information. One report indicated minor but persistent bleeding as a result of notching, but this has not been reported by others.
Freeze branding, using metal numerals 5-8 cm high applied to the animals’ body or dorsal fin for 10-20 sec, has been used safely and successfully with a variety of small cetaceans, including bottlenose dolphins, spinner dolphins, short-beaked common dolphins (Delphinus delphis), Pacific white-sided dolphins, short-finned pilot whales, false killer whales (Pseudorca crassidens), Amazon River dolphins, and rough-toothed dolphins (Steno bredanensis) (Irvine et al, 1982; Scott et al, 1990). Freeze-brand application typically results in little or no reaction by dolphins, but minor skin lesions may occur if brands are applied for too long. Readable white marks usually appear within a few days (Fig. 4). Freeze brands fade over time, but the marks can often still be identified for many years in good-quality photographs even if they are not readily visible in the field. Fading appears to be age related, with brands disappearing more rapidly and more completely on younger animals but remaining readable on adults for as long as 11 years or more (Irvine et al, 1982; Scott et al, 1990).
D. Attachment Tags
The use of attachment tags for identification purposes (rather than telemetry, covered elsewhere in this volume), including
Figure 4 (Top) Dorsal Jin of a 2-year-old female bottlenose dolphin showing a fresh freeze brand (“7″) above a year-old freeze brand and a 1-year-old roto tag. (Bottom) Dorsal fin of the same bottlenose dolphin at 5 years of age showing 3- and 4-ijear-old freeze brands, a naturally acquired notch at the top of the leading edge of the fin, and a notch formed from loss of a roto tag on the trailing edge of the fin.
Discovery tags, spaghetti tags, button tags, and roto tags, has been reviewed by Scott et al (1990). Discovery tags are numbered metal cylinders shot into the blubber from whaling ships or research vessels. The tags have been used primarily with baleen and sperm whales and are recovered when the whales are captured and rendered, providing information on two points within the animals’ range. Tagging was initiated in 1932 and continued until the whaling moratorium in 1985. More than 20,000 Discovery tags have been used, but return rates have been low, typically below 15%. Smaller versions of these tags have been used with small whales without notable success, and use with cetaceans less than 4.6 m long has been discouraged because of risk of serious injury.
Streamer or spaghetti tags, originally developed for fish tagging, are colored vinyl-covered strands of wire cable of variable length with steel or metal dart tips that are applied with either a crossbow or a jab stick, with the intent of anchoring the tip between blubber and muscle. Thousands of these tags have been applied to dolphins, porpoises, and belugas, especially in association with the tuna seine net fishery in the eastern tropical Pacific Ocean. Because of poor retention and high risk of injury to the animal, use of spaghetti tags with small cetaceans has been discouraged for many years (Irvine et al., 1982).
Dorsal fins or ridges are commonly used for tag attachment because of their structure, prominence, and regularity of appearance above the water’s surface. Button tags, typically numbered and colored fiberglass or plastic disks or rectangular plates designed after the Peterson disk fish tags, have been applied to several species of small cetaceans, including bottlenose dolphins, pantropical spotted dolphins, spinner dolphins, common dolphins, Pacific white-sided dolphins, belugas, and harbor poipoises (Evans et al., 1972; Scott et al., 1990). Usually, button tags are attached through the dorsal fin by means of one or more plastic (especially delrin) or stainless-steel bolts or pins that connect the tag halves on each side of the fin. Although some button tags have lasted for several years on pelagic dolphins, inshore animals often lose the tags within weeks or months, often by breaking them through rubbing on the shallow sea floor. Use of button tags has been largely discontinued due to poor tag retention and the potential for injury to the animals (Irvine et al., 1982).
Small plastic cattle ear tags, or rototags. clipped through the trailing edges of dorsal fins have proved successful for identifying small cetaceans in the field, including bottlenose dolphins, pantropical spotted dolphins, spinner dolphins, common dolphins, rough-toothed dolphins, Pacific white-sided dolphins, short-finned pilot whales, and harbor porpoises (Fig. 4a; Nor-ris and Pryor, 1970; Scott et al, 1990). Typically, a small hole is made in the thin tissue of the trailing edge using a sterile technique, and the tag is clipped through the fin with special pliers. Although the written markings are too small to be read at a distance, the number of tags, color, and position on the fin provide a useful degree of variation. Rototags have remained in position of periods of years, although often they are lost within months. Rototag halves may separate, leaving a healed hole in the fin, or they migrate through the trailing edge of the fin, leaving a small healed notch; both pose minimal risks to the animals but offer continuing identification features. Barnacle and algae fouling and pressure necrosis are infrequent problems. As a modification of this technique, small VHF radio transmitters have been attached to rototags for short-term tracking (up to 30 days), with a modification involving the use of a corrosi-ble nut system to release that tag at that time.
Other attachment techniques, such as the use of tethers or plastic-coated wires or polypropylene or soft rubber tubing, have proved to be ineffective and injurious to the animals when attached to the caudal peduncle. Tag loss rates have been high, and abrasions were frequently noted.
II. Pinnipeds
A. Natural Markings
Natural body markings have been used in only a few studies of pinnipeds such as gray seals (Halichoerus grypus), northern elephant seals (Miromga angustirostris), Steller sea lions (Eumetopias jubatus), Hawaiian monk seals (Monachus schauinslandi), harbor seals (Phoca vitulina), and California sea lions (Zalophus californianus). Yochem et al (1990) examined pelage patterns of harbor and larga (Phoca largha) seals to distinguish between populations and individuals. Using black and white photographs they scored the presence or absence of spots, clarity of spots, relative density of spots, complexity of spots, presence of rings, and spacing of rings in selected body areas (especially sides of the head, neck, and chest). Hiby and Lovell (1990) described a computer-aided matching system for screening a library of digitized natural mark photographs of gray seals. Their system created a three-dimensional model to locate features on the seal’s body, especially using the side of the neck. For most pinniped species, studies using natural markings are hampered by a lack of distinctive markings and the large numbers of individuals or pack ice distributions of many species (Erickson et al, 1993). Most pinniped researchers have resorted to the use of artificial markings and tags for individual identification.
B. Temporary Markings
Techniques for temporary markings of pinnipeds include paints, dyes, bleaches, and pelage clippings (Erickson et al, 1993). These techniques offer the advantages of often being able to be applied without having to restrain the animals and permitting remote identification without disturbance. However, these marks are typically lost upon moulting, precluding the continuity of identification beyond a single season. A variety of paints (marine, highway, rubber-based, quick-drying cellulose, aerosol sprays, and house paint) have been used to mark seals and sea lions. Paints have been applied from brushes or rollers on poles and from plastic bags thrown at the animals. Quick-drying paint has proved relatively effective, with a useful lifespan of about 1 month on average. Northern fur seals (Callorhinus ursinus) have been marked successfully for 2-12 months with a fluorescent plastic resin, naptha-based paint. This technique apparently results in the matting of guard hairs, which then break off. leaving an outline of the mark. High-gloss marine enamel applied from aerosol cans to mark Hooker’s sea lions (Phocarctas hookeri) has resulted in markings lasting 3 months, even after the animals have been at sea. Carbon dioxide-powered paint guns firing small capsules have proved less effective for marking elephant seals due to reliability problems and the small size of the marks.
Dyes have been used with several species of pinnipeds, especially light-colored species (Erickson et al, 1993). Successful dying usually occurs when permanent dyes are used and when the animals are dry and remain out of the water for a period of time following application. Colored dyes and black Nyanzol D have lasted 3-4 months on gray seals, harbor seals, and California sea lions. The addition of alcohol to Nyanzol D leaves a more distinct marker because it dissolves fur oils and also prevents the dye solution from freezing. Yellow picric acid in a saturated alcohol solution has been used with gray seals, with results that last through pup molting, appearing on the adults as well. This solution can be applied from a backpack tree sprayer to wet or drv seals. Fluorescent dye mixed with small quantities of epoxy resin has also been used with success. In some cases, such as southern elephant seals (Mirounga leonina), dyes have been less successful.
Bleach offers a very effective and sometimes longer-lasting alternative to paints and dyes (Erickson et al. 1993). Many of the bleach solutions can be applied to sleeping animals via a squeeze bottle, thus minimizing risk, effort, and disturbance. Commercially available products such as Lady Clairol Ultra Blue dye in combination with various chemicals have been used most often, resulting in a white or cream-colored mark that is most visible on dark pelages. Combinations resulting in thicker consistency allow for distinct lines. Bleach marks on elephant seals last until moult, sometimes for 6 months, and have lasted for two seasons on fur seals. Combinations of bleaches and dyes have also been used in some cases, such as northern elephant seals (Fig. 5).
Hair clipping is somewhat more difficult than the previous techniques, but effective when the underfur is a different color from the guard hairs (Erickson et al, 1993). This technique involves clipping or singeing the pelage to create a distinctive mark. It has been used with success with northern fur seals, Steller sea lions, and Antarctic fur seals (Arctocephalus gazella).
Figure 5 Bleach markings on a northern elephant seal. “Bilbo” is marked in black dye for identification through the summer molt and in bleach for the winter bleeding season.
C. Scarring and Branding
Punch marks and amputations have been used extensively with fur seals, with poor success and concerns about injury to the animals (Erickson et al, 1993). Initial efforts to mark northern fur seals and Antarctic fur seals by punching holes in flippers in unique combinations of numbers and positions found this technique to be unreliable due to healing and occlusion. Hair on the flippers of phocids seals precludes utility with these species. Flipper notching was also found to be unreliable due to tissue regrowth. Although ear notching was used successfully for cohort marking in northern fur seals, it is no longer used because of concerns regarding interference with diving abilities.
Both hot branding and freeze branding have been used with great effect with pinnipeds (Erickson et al, 1993). Hot brands have been used since 1912 with thousands of northern fur seals. Cape fur seals (A. pusillus), southern elephant seals, Weddell seals (Leptonychotes weddellii), grey seals, and leopard seals (Hy-drurga leptomjx). Some marks have remained readable for up to 20 years. The technique seems best suited to colonial seals due to the bulky nature of the branding tools and heat source. Typically, brands are heated to red hot and are applied with Ann, even pressure for 2-7 sec, depending on whether the hair has been clipped. Brands are applied to the upper saddle, middle back, or upper shoulder to optimize sightability.
Freeze branding differs from hot branding in that it involves the selective killing of pigment-producing cells through contact with a super-cooled metal numeral or symbol (typically 5 cm high) (Erickson et al, 1993). Brands are cooled with liquid nitrogen or a dry ice and alcohol mixture and applied for about 20 sec to an area where hair has been removed. Correct freeze brand application results in a nonpigmented pelage mark, ranging from dark (elephant seals, California sea lions) to pink (California sea lions; walrus, Odobenus rosmarus). Freeze branding has had mixed success. Many freeze brands on pinnipeds have been found to repigment within 1-2 years, perhaps as a result of excessive branding. Readable brands have been obtained for elephant seals (up to a year, discernible for 3 years), California sea lions (readable for 1.5 years, discernible for up to 4 years), walrus (readable for many years), and Australian sea lions, Neophoca cinerea (legible on flippers for 7 years, on flanks for 4 years).
D. Attachment Tags
Plastic or metal attachment tags are used more widely than any other kind of individual identification system with pinnipeds (Erickson et al, 1993). Monel or stainless-steel tags such as those used to mark livestock are the most common metal tags. These metal strap tags are self-piercing and are attached by means of special pliers to the trailing edge of the fore flippers of otariids and to the interdigital web of the hind flippers of phocids. Typically, the tags are stamped with an organization address and serial number. Thousands of metal tags have been attached to phocids. Retention rates on phocid seals are low, with postattachment tears and cuts sometimes becoming infected. Hundreds of thousands of metal tags have been attached to otariids, with similar poor results.
The use of plastic tags is now much more common than metal tags for identifying pinnipeds (Fig. 6). Two kinds of plastic bags are used commonly; rototags and Allflex tags. Both consist of self-piercing male and female elements that are applied with special pliers, as with metal tags. Plastic tags are available in a variety of colors, leading to more than 300 unique color combination possibilities. The visibility of both metal and plastic attachment tags can be enhanced through the use of streamer markers, such as nylon cloth strips reinforced with vinyl, which may last for a year or more.
Figure 6 Flipper tag on a northern elephant seal.
Tagging success with both metal and plastic tags is less than desired. Loss rates of the two kinds of plastic tags are variable, but tend to be lower than for metal tags, about 10% annually. However, the long-term durability of metal tags is better than plastic. Wounds from metal tags are more common than for plastic.
III. Sirenians
A. Natural Markings
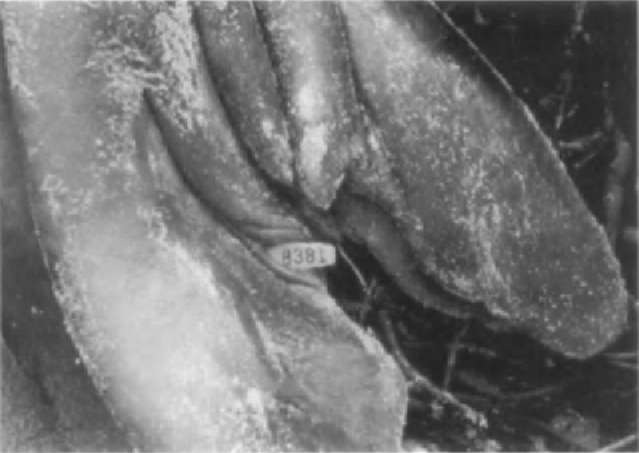
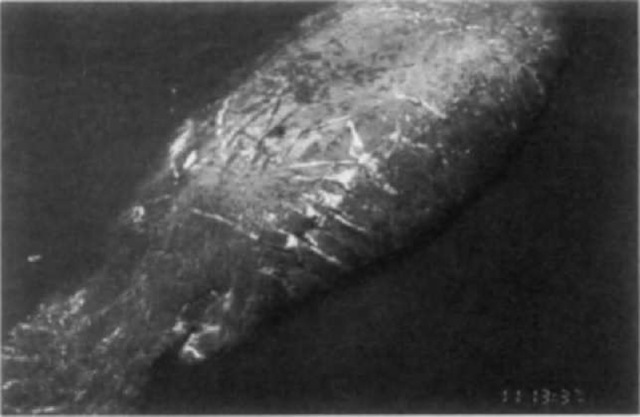
The process of developing new techniques and applying existing technology to studies of sirenians has been reviewed by G. Rathbun in Wells et al. (1999). Natural marks, including deformities and scars, have been used to identify individual manatees since the 1950s. Among the marks that have proven most useful for individual identification are the scars from collisions with boats, especially propeller scars. Most manatees (Trichechus manatus) in Florida waters bear scars from boat collisions, often from more than one event. Boat scars occur over all parts of the manatee’s body, but especially the dorsal surface and paddle, where notches may be cut by propellers (Fig. 7). Individual identification progressed from sketches of marks to surface and underwater 35-mm photography. Photography allowed for the tracking of changes in identifying characteristics through time and for distinguishing between manatees with similar markings. Technological advances have resulted in photographic images of scar patterns being saved, cataloged, and searched with the assistance of computers.
B. Temporary Markings
No widely accepted techniques currently exist for temporarily marking sirenians. Paint, flipper bands, and harnesses have been tested, but have been found to be ineffective (Irvine and Scott, 1984). “Paintstiks,” oil-based crayon-like markers, have remained visible for 3-7 days during field tests, although rubbing eventually smears or removes them. Aerosol paint was short-lived and application startled the animals and polluted the water.
C. Scarring and Branding
Although not intentional, the most widely used features for identifying individual manatees are propeller scars. In recent years, scientists have begun cutting small notches in the paddles of manatees. The positions of the notches around the paddle are coded to provide information on cohorts. Freeze branding is also used with manatees that have been captured or rehabilitated on occasion, with some success (Irvine and Scott, 1984). Although most brands fade with time, some have remained readable at distances of 15 m for as long as 4 years. Success may vary with whether the manatees are shedding, as well as season, water temperature, and salinity.
D. Attachment Tags
Lacking dorsal fins, sirenians provide few opportunities for tag attachment. As described for cetaceans, spaghetti tags have been tested with manatees (Irvine and Scott, 1984). These 20-cm-long plastic streamer tags attached to a metal dart have been applied with either a lance or a crossbow, attempting to anchor the tag about 2 cm below the skin. Spaghetti tags demonstrated poor retention and caused abscesses on some manatees.
The most effective technique for tag attachment involves a break-away “belt” looped around the animal’s peduncle. This belt is designed to minimize chafing, break away if it should become snagged on an obstacle in the environment, and carry a floating VHF or satellite-linked radio transmitter at the end of a tether. Each transmitter float is color coded to allow for individual identification visually. The tethers can be replaced by swimmers as necessarv.
Passive integrated transponders, or PIT tags, have been implanted in nearly every Florida manatee that has been handled in recent years (Wright et al, 1998). These glass-encapsulated microchips are about the size of a rice grain. They are implanted subcutaneously at a depth of about 3.5 cm, dorsal and caudal to the ear, and medial to the scapula. A small incision is made, and the tag is inserted via a 12-gauge needle. Each is programmed with a unique identification code that is activated by a hand-held scanner when it passes nearby. PIT tags are relatively easy to implant, last a long time, are reusable, rarely infect the animals, have an unlimited number of potential codes, and allow for easy data recording and transfer, but they suffer from the fact that they must be scanned from no more than 15 cm away and that the receivers are not waterproof.
Figure 7 Identifying scars from boat collisions on a Florida manatee. \