The bones and teeth of marine mammals, like those of other vertebrates, consist of both organic and mineral components. Because the mineral component (mostly calcium phosphate) predominates, the constituents of bones (bone and calcified cartilage) and teeth (cementum, dentine, and enamel) are referred to as “hard tissues.” Each of these hard tissues is distinguished both by its composition and by its microscopic structure. Many of the histological features of marine mammal teeth and bones are typical for mammals, and vertebrates, in general, but others are unique or unusual. Some of these may have evolved in conjunction with their shifts to marine habitats.
I. Bone
A. Bone Structure and Composition
Bone consists of highly calcified, intercellular bone matrix, and three types of cells: osteocytes, osteoblasts, and osteoclasts. The outer surface of bone is covered by periosteum, which is bound to bone by bundles of collagen fibers known as Sharpey’s fibers, and the inner bone surface is lined with endosteum (Fig. 1). Periosteum is thicker than endosteum, but bodi consist of fibrous connective tissue lined with osteoprogenitor cells from which osteoblasts are derived. Osteoblasts are the cells diat syn-diesize bone matrix proteins and are active in bone matrix mineralization. Bone matrix (also known as osteoid) consists of about 33% organic matter (mosdy type I collagen) and 67% inorganic matter (calcium phosphate, mostly hydroxyapatite crystals). Osteoblasts occur as a simple, epidielial-like layer at the developing bone surface. As the bone matrix mineralizes, some osteoblasts become trapped in small spaces within the matrix (lacunae). These trapped osteoblasts become osteocytes, the cells responsible for maintenance of the bony matrix. Each lacuna holds only a single osteocyte but is connected with adjacent lacunae by microscopic canaliculi, which house cytoplasmic processes of the osteocytes. Osteoclasts are large, multinucleated cells that occur in shallow erosional depressions (Howship’s lacunae) on the resorb-ing bone surface and secret enzymes that promote the local digestion of collagen and dissolution of mineral crystals.
Bone is commonly classified according to its gross appearance as cancellous bone (bone with numerous, macroscopic interconnecting cavities, or trabeculae, also known as spongy or trabecular bone) or compact bone (dense lamellar bone without trabeculae), but both types have the same basic histological structure. In a typical mammalian long bone the diaphysis (shaft) is composed predominantly of compact bone, with cancellous bone confined to the inner surface around a central, medullary cavity (Fig. la), whereas the epiphyses (articular ends) consist mostly of cancellous bone overlain by a thin, smooth layer of compact bone. In short bones a core of cancellous bone is completely surrounded by compact bone, and in the flat bones of the skull, inner and outer plates of compact bone are separated by the diploe, a layer of cancellous bone.
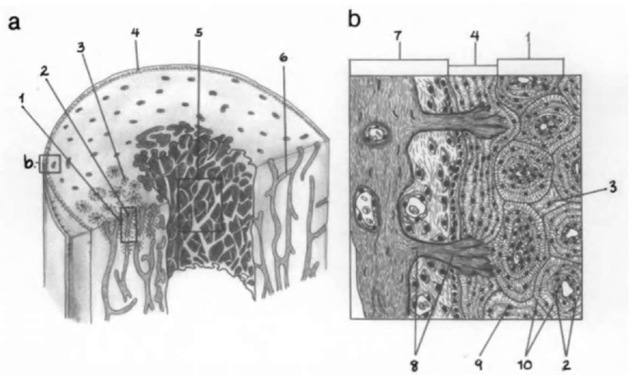
Bone also can be classified histologically, as woven (primary) bone and lamellar (secondary) bone. Woven bone, or primary bone, has an irregular structure and is usually replaced in adults by the more highly mineralized lamellar bone. Lamellar bone is organized into thin layers (lamellae), usually 3-7 |xm thick, which contain parallel collagen fiber bundles. Lacunae containing osteocytes are located between lamellae. There are three types of lamellae: concentric, interstitial, and circumferential (Fig. lb). Concentric lamellae are arranged in circular layers around a long axis, the haversian canal, which is a vascular channel containing blood vessels, nerves, and connective tissue. Adjacent vertical channels are connected by more horizontally oriented vascular channels (Volkmann’s canals). The entire complex, consisting of several layers of concentric lamellae around a vascular channel, is known as an osteon or haversian system. Interstitial lamellae, which appear as irregularly shaped areas between adjacent osteons, consist of lamellae that are remnants of osteons destroyed during bone remodeling. Circumferential lamellae are arranged parallel to each other and comprise the outer circumferential lamellae laid down next to the periosteum and the inner circumferential lamellae laid down next to the endosteum.
Figure 1 (a) Schematic model of the ivall of a mammalian long-bone diaphysis consisting of an outer layer of compact bone and an inner lay er of cancellous bone, surrounding a central medullary cavitij. Periosteum covers the outer bone surface, and endosteum covers the inner bone surface, (b) Enlarged diagram of periosteum and compact bone in (a) 1, osteon; 2, haversian canal; 3, interstitial lamellae; 4, outer circumferential lamellae; 5, cancellous bone; 6, Volkman’s canal; 7, periosteum; 8, Sharpey’s fibers; 9, lacuna; 10, concentric lamellae. Adapted from Ten Cate (1989).
B. Bone Formation, Growth, and Remodeling
Osteogenesis (bone formation) of membrane bone (in-tramembranous bone, dermal bone) occurs directly by the mineralization of matrix formed by osteoblasts within condensations of mesenchyme. Osteogenesis of endochondral bone (cartilage bone) occurs indirectly by the deposition and mineralization of bone matrix on a preexisting cartilage matrix. Bone continues to grow through remodeling, as old bone is resorbed through activities stimulated by osteoclasts, and new bone is laid down through the activities of osteoblasts. Influences on bone remodeling include strain and stress imposed by movement and muscle action, hormones, and growth factors. For example, the parathyroid hormone is linked to osteoclast proliferation and activity, and calcitonin, a hormone synthesized within the thyroid, has an inhibitoiy effect on osteoclast activity (Marks and Popoff, 1988). Modifications of hormonal controls on bone growth and remodeling, in specific parts of the skeleton, are probably responsible for specializations in bone density patterns in some marine mammals (see later). In addition to this typical osteoblastic bone formation, osteogenesis can occur through the direct transformation of cartilage or fibrous tissue into bone (metaplastic bone). The metaplastic transformation of chondrocytes into osteoblasts may account for the formation of osseous globules found in the endosteal endochondral bone of some archaeocetes (Buffrenil et al, 1990). Osseous globules are pseudolamellar bone deposited in empty lacunae that once housed chondrocytes, but their mode of origin is controversial.
Because bone growth occurs throughout life, periodic growth marks in skeletal tissue, particularly periodical deposition of periosteal bone layers, are potentially useful in mammalian age determination. Techniques of skeletal tissue age determination involve the counting of growth layer groups. Growth layer groups are sets of incremental growth lines defined by at least one change in mineral density, such as between more stained and less stained layers or dark and light layers. However, the dynamic nature of bone growth and remodeling limits the accuracy of bone growth layer group counts.
C. Marine Mammal Bone
Marine mammals show two very different trends in bone architecture and histology, reduced bone density and increased bone density, both of which are associated with their aquatic habits (Wall, 1983). Deep-diving marine mammals, especially Recent cetaceans, have bones that are less dense than homologous elements in terrestrial mammals. They efficiently overcome buoyancy at depth by the active mechanism of lung collapse. whereas at the surface their lighter bones enhance buoyancy, allowing them to float with relatively little expenditure of energy. A pattern of reduced bone density has been documented thoroughly in small to medium-sized odontocetes. some of the large-bodied cetaceans, and some phocids (notably the elephant seals. Mirounga spp.) and is characterized by the replacement of cortical bone (the compact bone surrounding medullary cavities) with cancellous bone, which also fills the medullary cavities. This condition is apparently caused by an imbalance between bone resorption and redeposition beginning early in ontogeny and is probably under hormonal control. An increase in cancellous bone in these mammals does not appear to be pathological—the microscopic architecture of cancellous bone in cetacean limbs is significantly more organized than that of typical osteoporotic bone.
In contrast, shallow-diving marine mammals, such as sirenians. overcome buoyancy while diving in large part by the static mechanism of increased bone density. Their bones are much denser than typical mammal bones. This is achieved in different ways: by osteosclerosis, by pachyostosis, or by a combination of both conditions (pachvosteosclerosis) (Domning and Buffrenil, 1991). Sirenians show pronounced pachyosteosclero-sis, especially in the thoracic and occipital regions. Similarly, walruses and some seals have unusually dense limb bones.
Ongoing research in the bone histology of extinct marine mammals indicates that both increased bone density and reduced bone density have evolved independently several times in different groups of marine mammals. The earliest sirenians show pronounced pachyosteosclerosis. Likewise, in contrast to Recent cetaceans, some bones of extinct Eocene archaeocetes are osteosclerotic. In Basilosaurus (Basilosauridae), the osteosclerosis of ribs is very pronounced, with the total replacement of medullary trabecular bone by compact bone (Buffrenil et al, 1990). Similarly, hyperostosis of the periosteal cortex and infilling of the medullary cavity with cancellous bone occur in ribs and vertebrae of Eocetiis (Protocetidae) (Uhen, 1999) and in ribs of Zygorhiza (Durodontidae) (Buffrenil et al, 1990). In contrast, long bones of some other durodontids show a reduced diickness of periosteal compact bone, as in modem cetaceans (Madar, 1998).
Bone of one marine mammal, the toothed whale Mesoplodon densirostris, exhibits unique histological features. The rostral bone of this odontocete, which is among the densest bone known among tetrapods, is characterized by hyperminer-alized secondary osteons. These osteons have unusually well-aligned parallel and platy hydroxyapatite crystals and a tubular network of unusually thin collagen fibrils, and thus differ markedly from the structure of haversian systems of typical mammalian lamellae bone (Zylberberg et al, 1998).
The periodic deposition of periosteal bone layers has been used in studies of age determination in mammals, although limited in use by the fact that mammalian bone undergoes remodeling throughout life (Klevezal. 1996). Bone growth layer groups have been studied in a variety of marine mammals, including sirenians, pinnipeds, and odontocetes.
II. Cementum
A. Cementum Structure and Composition
Teeth of marine mammals, like all mammals, consist of a crown, which extends above the gums, and one or more roots, which extend below the gum line and hold the teeth in bony sockets (alveoli). The roots are covered by cementum (also known as cement), which sometimes extends to cover part of the crown, overlapping the cervical enamel. Cementum, along with the periodontal ligament, comprises the periodontium, the attachment apparatus of teeth. Cementum is similar in composition to bone. Its mineral component (65% by wet weight) consists of crystals of an impure form of hydroxyapatite similar in shape and size to those of bone. Its organic component (up to 20% of the total tissue) includes cementocytes (cementum cells), ground substance containing proteoglycans, intrinsic collagen fibers, and extrinsic collagen fibers (Sharpey’s fibers). Intrinsic fibers and ground substance are the primary constituents of cementum. Intrinsic fibers, like collagen fibers of lamellar bone, are small, on the order of 1-2 |xin in diameter. The extrinsic Sharpey’s fibers are much larger, typically 3-12 |xm in diameter. Intrinsic fibers, ground substance, and cementocytes are derived from cementoblasts, but the extrinsic fibers are derived from fibroblasts of the periodontal ligament.
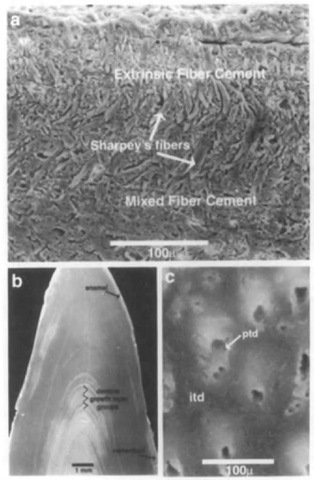
Cementum is classified according to the relative proportions of the different components, although the different types are gradational. Thus, cementum can be classified as cellular or acellular depending on the relative proportions of cementocytes and ground substance, or it can be classified according to its fiber composition (Fig. 2). Extrinsic fiber cement occurs close to die alveolar bone and is dominated by well-mineralized Sharpey’s fibers contained within a highly mineralized acellular ground substance. Mixed fiber cement contains intrinsic collagen fibers as well as Sharpey’s fibers and ground substance and may contain cementocytes. Intrinsic fiber cement, which contains only intrinsic collagen fibers, ground substance, and cementocytes, occurs close to roots. In cellular mixed fiber cement and intrinsic fiber cement, cementocytes are contained in lacunae of variable shape that form within the mineralizing ground substance.
Incremental growth layers known as cementing lines or resting lines are sometimes a prominent histological feature of both cellular and acellular cementum. Cementing lines, like the incremental growth layers found in bone, dentine, and enamel, are distinct layers that parallel the developing surface. Due to periodic variation in mineralization during development, they contrast with adjacent layers. Cementum growth layer groups, like those of bone and dentine, can be defined empirically by at least one change in mineral density, such as between translucent and opaque layers, dark and light layers, ridge and groove, or more stained and less stained layers. Empirical studies have shown that cementum growth layer groups record the periodicity of tissue formation and thus are useful in age determination.
B. Marine Mammal Cementum
Cementum in marine mammals is, for the most part, structurally similar to that of other mammals. Cementum growth layer groups are used in conjunction with dentine and bone growth layer groups to estimate age in marine mammals, although their relative clarity varies among species. In some species, cementum formation continues beyond that of dentine, which is an advantage in age determination. In ziphiid whales, where the cementum typically extends over most of the crown and may comprise the bulk of the tooth, cementum growth layer groups are distinguishable without magnification. Ziphiids also have been reported to have an unusual, possibly vascular cementum (Boyde, 1980).
Figure 2 Scanning electron micrographs of an isolated tooth of an unidentified delphinoid cetacean (Yorktown Formation, Pliocene, from the Lee Creek Mines, North Carolina). The specimen has been sectioned longitudinally, polished, and etched with dilute HCl (a) High magnification view of cementum, which grades from extrinsic fiber cement on the outer periphery/ (top) to mixed fiber cement closer to the cementum-dentine junction (bottom). Classification of cementum depends on the proportion of Sharpey’s fibers contained within the matrix, (b) Thin layers of enamel and cementum lie peripheral to dentine of the crown and root. Dentine growth layer groups appear as pairs of dark/light bands, (c) High magnification view of dentine. The walls of cross-sectioned dentine tubules contain hy-permineralized peritubular dentine (ptd). Less mineralized in-tertubular dentine (itd) occurs between tubules.
III. Dentine
A. Dentine Structure and Composition
Dentine comprises the bulk of the volume of teeth of most mammals. In the crown, dentine is covered by enamel, whereas in the root it is covered by cementum. Circumpulpal dentine surrounds the pulp cavity, which contains connective tissue, nerves, and blood vessels. Circumpulpal dentine is distinguishable histologically from a thin outer layer known as mantle dentine (in the tooth crown) or hyaline dentine (in the root). Dentine tubules, which radiate out from the pulp to the outer dentine surface, are distinctive features of dentine. They are narrow (1—4 |xm diameter) tubular structures that form during dentine development around odontoblast (dentine-forming cells) cell processes. In adult teeth, tubules contain mostly fluid and amorphous cell debris.
The organic component of dentine consists mainly of veiy small (on the order of 50 nm in diameter) collagen fibrils. The collagen fibrils in circumpulpal dentine are laid down parallel to the developing dentine surface and perpendicular to the dentine tubules, but the mantle dentine contains some large (more than one micron in diameter) collagen fiber bundles known as von Korff fibers. Von Korff fibers are oriented parallel to tubules.
Dentine is 75% mineral (hydroxyapatite). Most of the small (2-3 nm in thickness and probably 20-100 nm in length) hydroxyapatite crystals are aligned parallel to each other and to the small collagen fibrils, but others are oriented radially and form spherical or semispherical structures known as calcos-pherites. Calcospherites are difficult to distinguish histologically because they typically fuse together. Areas where mineralization is incomplete and calcospherites have not fused are called interglobular dentine. Most mineralization of dentine takes place along the developing dentine front, but dentine deposited in tubule walls (peritubular dentine) undergoes further mineralization (Fig. 2c). In some cases, tubules become occluded by mineralization, forming sclerotic dentine. Denticles (smooth-surfaced, spherical mineralized bodies with a laminar structure) sometimes form by the mineralization of collagen fibers within the pulp cavity. These denticles may become attached to the inner surface of the dentine or become embedded in it during continued dentine formation.
B. Marine Mammal Dentine
The dentine of most marine mammals is structurally similar to that of other mammals, but there are some exceptions. In some, notably the narwhal (Monodon monoceros) and sperm whale (Physeter niacrocephalus), the large von Korff fibers are not restricted to the mantle dentine but extend throughout the thickness of dentine, where they are located in the walls of dentine tubules. Denticles have been reported in some odontocetes and sclerotic dentine is found in some marine mammals, especially in seals.
Marine mammal dentine is characterized by prominent incremental growth layers (Fig. 2b) that lie at angles to dentine tubules and vary in their intensity, both within and among individuals. The finest scale layers are the incremental von Ebner lines, which probably reflect diurnal variation in matrix fiber arrangement. Von Ebner lines appear as alternating dark and light lines in ground sections under polarized light. Other larger-scale incremental growth layers reflect changes in density due to differences in mineralization. These include the neonatal line, a very prominent growth layer that marks physiological disturbance associated with birth, and other less distinct and consistent growth layers whose physiological bases are uncertain. In some seals, the growth layer groups are accentuated by layers of interglobular dentine. Whatever their origins, there is a regular repetition to growth layer groups that seems to reflect annual or semiannual growth cycles, and counting of dentinal growth layer groups is a primary basis of age determination in pinnipeds, sirenians, and odontocetes.
IV. Enamel
A. Enamel Structure and Composition
Enamel covers the tooth crown in most mammals. It is the most highly mineralized tissue in the body, consisting almost entirely (95% by weight) of highly structured arrangements of hydroxyapatite crystallites. The remaining fraction consists of water and two classes of proteins unique to enamel: enamelins, which predominate in mature (fully mineralized) enamel, and amelogenins, which predominate in developing enamel. The histological structure of enamel reflects the organization of crystallites into units of increasing scale, two of which are enamel prisms and enamel types. This structural organization is determined during enamel development. Unlike bone, cement, and dentine, enamel does not remodel after its initial deposition.
Enamel matrix is secreted by ameloblasts. The activity of these enamel-secreting cells commences at the enamel-dentine junction (EDJ) and continues as ameloblasts retreat outward, away from the EDJ. Mineral crystals precipitate and grow within the enamel matrix left by the retreating ameloblasts. The orientation and arrangement of the crystallites, and thus the structure of the mature enamel, depend on the shape of the secretory end of the ameloblast. The simplest enamel structure is formed by ameloblasts with flat secretory surfaces. In most mammal teeth, however, the bulk of the enamel is laid down by ameloblasts whose secretory ends form protrusions, called Tomes processes, surrounded by flattened areas called ameloblast shoulders. Because enamel crystallites grow perpendicular to the differently oriented secretory surfaces of the Tomes process and the ameloblast shoulder, there is a regular pattern of discontinuities in crystallites’ orientations. These discontinuities define the boundaries of enamel prisms and interprismatic enamel.
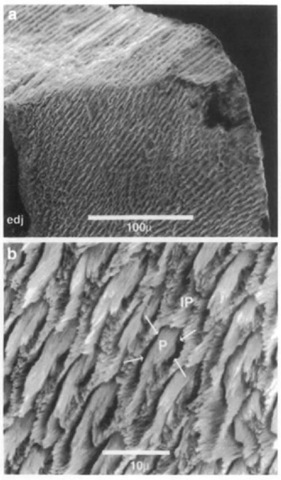
Enamel prisms are cylindrical bundles of largely parallel hydroxyapatite crystals extending outward from the EDJ toward the outer tooth surface. The prism boundaries are defined by differences in orientations between prismatic crystallites and those of the adjacent enamel that fills the spaces between prisms. This enamel is called interprismatic enamel. It is com-positionally identical to enamel prisms, but differs in crystallite orientation. The submicroscopic gap produced by the change in crystallite orientations at the prism-interprismatic boundary is known as the prism sheath (Fig. 3). Prism sheaths contain slightly greater concentrations of water and protein than the surrounding enamel, and thus are less dense. This allows prism patterns (the cross-sectional shapes and packing arrangement of prisms and interprismatic enamel) to be distinguished in ground sections or in acid-etched scanning electron microscope preparations. Prisms may have closed, circular cross sections or open, arc-shaped cross sections. Prism patterns have been used to distinguish among some mammalian groups, but there is considerable variation within individuals, and considerable parallelism among different groups.
Figure 3 (a) Scanning electron micrograph of fractured enamel near tip of tooth (unidentified Pliocene odontocete, Lee Creek Mine, North Carolina). The naturally fractured surface (at top) shows that the prisms take a straight course from the enamel-dentine junction (edj) to the outer surface, as is typical in radial enamel, (b) High magnification view showing enamel prisms (P) sectioned oblique to their long axes. Prism crystallites are parallel to each other, but not to crijstaUites in adjacent interprismatic enamel (IP). Arrows indicate the position of the prism sheath, which has been artificially enlarged in this acid-etched specimen.
Enamel types describe the organization of enamel at a scale greater than individual crystallites or prisms. Common enamel types include parallel crystallite enamel, radial enamel, and decussating enamel. Parallel crystallite enamel, a type of nonpris-matic enamel, is a volume of enamel in which hydroxyapatite crystallites are parallel to each other with no discontinuities in orientation and lacking larger-scale structural features, other than incremental lines. Radial enamel refers to a volume of prismatic enamel where prism long axes are parallel to one another and directed radially outward from the EDJ. Decussating enamel is a volume of enamel characterized by layers of parallel prisms, one or more prisms in thickness, whose long axes alternate in orientation with prisms in adjacent layers. Decussating enamel, also known as Hunter-Schreger bands (HSB), includes undulating HSB, where layers of similarly oriented prisms have a gently undulating course from the EDJ to the surface, and zigzag HSB, where the layers undulate with a pronounced vertical amplitude. Differences in enamel types have a phylogenetic component, but also have different mechanical properties that can be important functionally—parallel crystallite enamel may be harder than prismatic enamel, but prismatic enamel, especially decussating enamel, is more resistant to cracks induced by chewing stress. Zigzag enamel is thought to be especially resistant to cracking. Most mammal teeth are composed of more than one enamel type.
Cross-striations, a record of the daily incremental deposition of enamel, are sometimes evident in both prismatic and non-prismatic enamel. In the scanning electron microscope, cross-striations appear as alternating constrictions and varicosities along the length of the prism, suggesting that they reflect variations in the rate of enamel secretory activity. More prominent incremental lines, known as brown striae of Retzius, also transect prisms or crystallites. They are oriented parallel to the developing enamel surface and probably reflect regular interruptions in growth, although their causes and periodicity are not clear.
B. Marine Mammal Enamel
Although the crowns of most marine mammal teeth are covered with enamel, there is considerable variation in its structural complexity among and within orders. Likewise, prism patterns vaiy among and without orders, although there is no compelling evidence that prism patterns are diagnostic of particular marine mammal groups.
Most extant cetaceans have thin, structurally simple enamel. In some the enamel consists of a thin layer of radial prismatic enamel with or without an outer layer of nonprismatic parallel crystallite enamel, and in many species the tooth enamel consists entirely of nonprismatic parallel crystallite enamel. In contrast, the most primitive cetacean, the fossil Pakicetus, had relatively thick enamel with a more complex structure consisting of parallel crystallite enamel, radial enamel, and a thick inner layer of undulating Hunter-Schreger bands. Later archaeocetes show the same arrangement of enamel types, but almost all more derived odontocetes have much less complex enamel.
This has led some workers to conclude that the enamel of most extant cetaceans is evolutionarily degenerate. Only two extant odontocetes, the Indian river dolphin Platanista gangetica and Amazon dolphin Inia geoffrensis have well-developed, undulating HSB. It is unclear whether these were acquired independently in response to functional demands of their diet or a primitive retention from archaeocete ancestors.
Extant sirenians (Dugong dugon and Trichechus spp.) are reported to have radial enamel with variably circular and arc-shaped prism cross sections. Similar enamel has been reported for some fossil sirenians, and it is likely that this is primitive for the group. Pinniped enamel has not been described in detail, but enamel of some species appears to be more complex than that of sirenians. Phoca vitulina has undulating HSB, and walrus (Odobenus rosmarus) enamel shows a transition from undulating HSB to zigzag HSB near cusp tips.
Enamel incremental lines generally are not used in the age determination of marine mammals. The thin enamel of many species makes resolution of these lines difficult and, more importantly, enamel only records the period of tooth development during which enamel is laid down, which, is most cases, is before birth.