I. Diagnostic Characters and Taxonomy
Baleen or whalebone whales (Mysticeti) comprise one of the two recent (nonfossil) cetacean suborders. They differ from the other suborder (toothed whales, Odonto-ceti), particularly in their lack of functional teeth. Instead they feed on relatively very small marine organisms by means of a highly specialized filter-feeding apparatus made up of baleen plates (“whalebone”) attached to the gum of the upper jaw.
Other differences from toothed whales include the baleen whales’ paired blowhole, symmetrical skull, and absence of ribs articulating with the sternum.
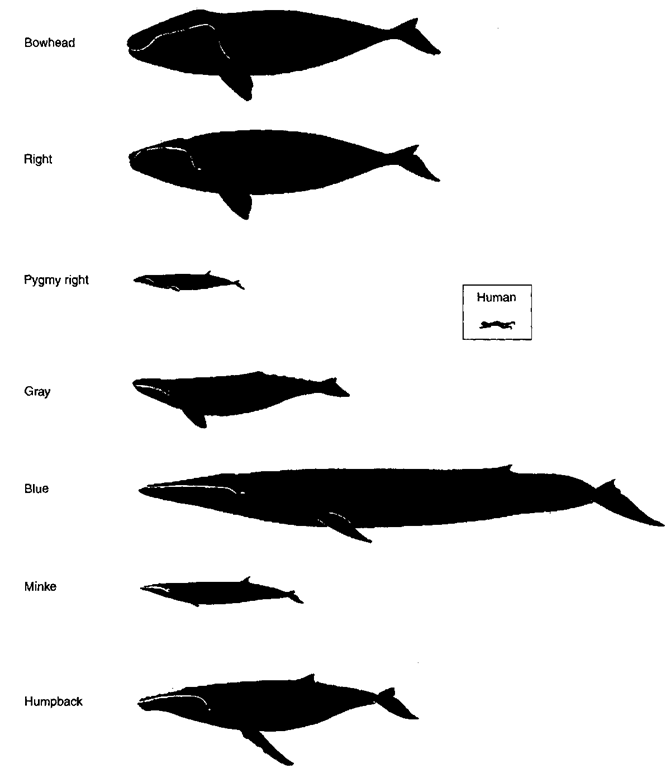
Baleen whales are generally huge (Fig. 1). In the blue whale they include the largest known animal, growing to more than 30 m long and weighing more than 170 tons. Like all other cetaceans, baleen whales are totally aquatic. Like most of the toothed whales, they are all marine. Many undertake very long migrations, and some are fast swimming. A few species come close to the coast at some part of their life cycle and may be seen from shore; however, much of their lives is spent remote from land in the deep oceans. Baleen whale females grow slightly larger than the males. Animals of the same species tend to be larger in the Southern than in the Northern Hemisphere.
Within the mysticetes are four families: right whales (Bal-aenidae, balaenids), pygmy right whales (Neobalaenidae, neobalaenids), gray whales (Eschrichtiidae, eschrichtiids), and “rorquals” (Balaenopteridae, balaenopterids). Within the suborder, 13 species are now generally recognized (Table I).
Right whales are distinguished from the other three families by their long and narrow baleen plates and arched upper jaw. Other balaenid features include, externally, a disproportionately large head (ca. one-third of the body length), long thin rostrum, and huge bowed lower lips; they lack multiple ventral grooves.
TABLE I Mysticetes (Baleen Whales)
|
|
|
|
|
|
Maximum |
|
|
|
|
|
|
|
length |
|
|
Family |
Genus |
Species |
Subspecies |
Common name |
(m) |
Generalized distribution |
|
Balaenidae |
|
|
|
Right whales |
|
|
|
|
Balaena |
B. mysticetus |
|
Bowhead whale |
19.8 |
Circumpolar in the Arctic |
|
|
Eubahena |
E. glacialis |
|
North Atlantic right whale |
17.0 |
Temperate-Arctic |
|
|
|
E. australis |
|
Southern right whale |
17.0 |
Temperate-Antarctic |
|
|
|
E. japonica |
|
North Pacific right whale |
17.0 |
Temperate N. Pacific |
|
Neobalaenidae |
|
|
|
Pygmy right whales |
|
|
|
|
Neobalaena |
Caperea marginata |
|
Pygmy right whale |
6.4 |
Temperate, Southern |
|
|
|
|
|
|
Hemisphere only |
|
|
Eschrichtiidae |
|
|
|
Gray whales |
|
|
|
|
Eschrichtius |
E. robustus |
|
Gray whale |
14.1 |
North Pacific-Arctic |
|
Balaenopteridae |
|
|
|
Rorquals |
|
|
|
|
Megaptera |
M. novaeangliae |
|
Humpback whale |
16.0 |
Worldwide |
|
|
Balaenoptera |
B. acutorostrata |
|
Common minke whale |
|
Worldwide |
|
|
|
|
B. a. acutorostrata |
N. Atlantic minke whale |
9.2 |
Temperate-Arctic |
|
|
|
|
B. a. scammoni |
N. Pacific minke whale |
p |
Temperate-Arctic |
|
|
|
|
B. a. subsp. |
Dwarf minke whale |
? |
Temperate-sub-Antarctic, |
|
|
|
|
|
|
Southern Hemisphere |
|
|
|
|
|
|
|
only |
|
|
|
|
B. bonaerensis |
|
Antarctic minke whale |
10.7 |
Temperate-Antarctic |
|
|
|
B. edeni |
|
Brydes whale |
14.0 |
Circumglobal, tropical- |
|
|
|
|
|
|
subtropical |
|
|
|
|
B. borealis |
|
Sei whale |
17.7 |
Worldwide, largely |
|
|
|
|
|
|
temperate |
|
|
|
|
B. physalus |
|
Fin whale |
26.8 |
Worldwide |
|
|
|
B. musculus |
|
Blue whale |
|
Worldwide |
|
|
|
|
B. m musculus |
Blue whale |
26.0 |
N. Atlantic, N. Pacific |
|
|
|
|
B. m. indica |
Great Indian rorqual |
p |
N. Indian Ocean |
|
|
|
|
B. m. brevicauda |
Pygmy blue whale |
24.4 |
Southern Hemisphere, |
|
|
|
|
|
|
temperate-sub-Antarctic |
|
|
|
|
|
B. m. intermedia |
“True” blue whale |
30.5 |
Southern Hemisphere, |
|
|
|
|
|
|
temperate-Antarctic |
Figure 1 Lateral profiles of representative baleen whales, with a human figure, to scale.
Internally, there is no coronoid process on the lower jaw and cervical vertebrae are fused together. Within the family are two distinct groups: the bowhead (Balaena mysticetus) of northern polar waters (formerly known as the “Greenland” right whale) and the three “black” right whales (Eubalaena spp.) of more temperate seas (so called to distinguish them from the “Greenland” right whale). All balaenids are robust.
Pygmy right whales (Caperea marginata) have some features of both right whales and balaenopterids. The head is short (ca one-quarter of the body length), although with an arched upper jaw and bowed lower lips, and there is a dorsal fin. The relatively long and narrow baleen plates are yellowish-white, with a dark outer border, quite different from the all-black balaenid baleen plates. Internally, pygmy right whales have numerous broadened and flattened ribs.
Gray whales (Eschrichtius robustus) are also somewhat intermediate in appearance between right whales and balaenopterids. They have short narrow heads, a slightly arched rostrum, and between two and five deep creases on the throat instead of the balaenopterid ventral grooves. There is no dorsal fin, but a series of 6 to 12 small “knuckles” along the tail stock. The yellowish-white baleen plates are relatively small.
Balaenopterids comprise the six whales of the genus Balaenoptera (blue, B. musculus; fin, B. physalus; sei, B. borealis; Bryde’s, B. edeni; common minke, B. acutorostrata and Antarctic minke, B. bonaerensis) and the humpback whale (Megaptera novaeangliae). All have relatively short heads, less than a quarter of the body length. In comparison with right whales, the baleen plates are short and wide. Numerous ventral grooves are present, and there is a dorsal fin, sometimes rather small. Internally, the upper jaw is relatively long and unarched, the mandibles are bowed outward, and a coronoid process is present; cervical vertebrae are generally free. All seven balaenopterids are often known as “rorquals” (said to come from the Norse “whale with pleats in its throat”). Strictly speaking, the term should probably be applied to the six Balaenoptera species, recognizing the rather different humpback in its separate genus, but many authors now use it for all seven balaenopterids.
Baleen whales are sometimes called “great whales.” Despite their generally huge size, some of the species are relatively small, and it seems preferable to restrict the term to the larger mysticetes (blue, fin, sei, Bryde’s, humpback) together with the largest odontocete (the sperm whale, Physeter macrocephalus).
In a recent review of the systematics and distribution of the world’s marine mammals, Rice (1998) has drawn attention to the derivation of the Latin word Mysticeti and clarified the status of a variant, Mystacoceti. He describes the former as coming from Aristotle’s original Greek mustoketos, meaning “the mouse, the whale so-called” or “the mouse-whale” (said to be an ironic reference to the animals’ generally vast size). Mystacoceti means “moustache whales,” and although used occasionally in the past (and more obviously appropriate for whales with baleen in their mouths) it has been superseded by Mysticeti.
The 13 species in Table I differ somewhat from those listed by Rice. Some authors disagree with his use of the genus Balaena for Eubalaena and his preference for the single species glacialis rather than the three species, Eubalaena glacialis, E. japonica, and E. australis (the North Atlantic, North Pacific and southern right whales). While acknowledging the need for further investigation, they refer to present-day biologists’ usage, and genetic information, in preferring a separation between Northern and Southern Hemisphere animals and in recognizing two species in the northern hemisphere; Eubalaena is, however, the only mysticete genus where one or more separate species is recognized in each hemisphere. Rice also distinguishes between two species of Bryde’s whale: Balaenoptera edeni and B. brydei. The taxonomie status of these “inshore, smaller” and “offshore, larger” forms has yet to be determined and here they are subsumed within B. edeni. In the case of the blue whale, Rice’s inclusion of a northern Indian Ocean form (B. m. indiea, referred to by Rice as “the great Indian rorqual”) has been followed. Similarly, his listing of three subspecies of minke whale, including the Southern Hemisphere dwarf minke, which has yet to be formally described, has been retained. However, other subspecies, e.g., two sei whales and two fin whales, have not been included.
II. Distribution and Ecology A. Habitat
In addition to the subspecies listed in Section I, many stocks or populations have been recognized, some mainly for management purposes, based on more or less valid biological grounds. Some significant examples include:
1. Bowhead Whales In addition to the currently most abundant population (the Bering-Chukchi-Beaufort Seas stock), four others are recognized: Baffin Bay/Davis Strait, Hudson Bay, Spitzbergen, and Okhotsk Sea.
2. Right Whales In the North Atlantic species, two populations are currently recognized, western and eastern, with calving grounds off the southeastern United States and northwestern Africa. The latter may now represent only a relict populations). In the North Pacific species, the current view is that there well may once have been two or more stocks, based on feeding ground information: at least one now centered in summer on the Sea of Okhotsk and another, although possibly not now a functioning unit, summering in the Gulf of Alaska.
In the southern right whale, there are several populations, defined by currently occupied calving grounds, but these cover only a proportion of the many areas known from historical whaling records to have once been occupied by right whales. Up-to-date information is available on presumed discrete populations off eastern South America, South Africa, southern Australia, and sub-Antarctic New Zealand.
3. Gray Whales A western North Atlantic population may have persisted until the 17th or 18th centuries, but is now extinct. The species now survives only in the North Pacific, where, in addition to a flourishing “Californian” stock, wintering on the coast of Baja California, and summering in the Bering Sea, animals are now being reported from a remnant western stock, summering in the northern Okhotsk Sea.
Figure 2 Head of a right whale showing the arrangement of the filter-feeding apparatus.
4. Humpback Whales In the North Atlantic, two major populations are recognized: one based on animals wintering in the West Indies and the other, now possibly only a relict population, wintering around the Cape Verde Islands. In the North Pacific, three discrete wintering grounds have been recorded: around the Bonin, Mariana, and Marshall Islands in the west; around the Hawaiian Islands in the center; and off Mexico in the east.
In the Southern Hemisphere, seven populations have been postulated. Six are well defined, based on calving (wintering) grounds on either side of each continent (one off eastern Australia is closely related to animals wintering off Fiji and Tonga), and a possible seventh in the central Pacific. In the northwest Indian Ocean, there seems to be a separate population where animals have been reported present throughout the year.
Baleen whales thus occupy a wide variety of habitats, from open oceans to continental shelves and coastal waters, from the coldest waters of the Arctic and Antarctic, through waters of both hemispheres and into the tropics.
Most specialized is the bowhead, Balaena, restricted to the harsh cold and shallow seas of the Arctic and sub-Arctic. The black right whales (Eubalaena) are more oceanic and prefer generally temperate waters, but come very close to coasts in winter to give birth, particularly in the Southern Hemisphere. Once believed not to penetrate much further south than the Antarctic convergence (ca 50-55°S), there have been recent records in the Antarctic proper, south of 60°S. Whether this is a new phenomenon is unclear: a report by Sir James Clark Ross of many “common black” (i.e., right) whales in the Ross Sea (eastern Antarctic) at 63°S in December 1840 was discounted when their presence there later that century could not be confirmed. It has been suggested that the currently greatly reduced population of the western North Atlantic right whale, now wintering off the southeastern United States and summering in coastal waters north to the Bay of Fundy (ca 45°N), may represent the peripheral remnant of a more widely distributed stock, formerly summering north to Labrador and southern Greenland, i.e., to at least 60°N.
The pygmy right whale (Caperea) is restricted to Southern Hemisphere temperate waters, between about 30 and 52°S; it can be found coastally in winter in some areas, and all-year round in others.
Gray whales (Eschrichtius) are the most obviously coastal baleen whales. The long coastal migration of the “Californian” stock, from Mexico to Alaska, supports a major whale-watching industry from December to April. In spring the animals migrate through the Bering Strait into the more open waters of the Bering Sea, but still favoring more shallow waters.
Among the balaenopterids, fin and sei whales are probably the most oceanic, with the former penetrating into colder waters than the latter in summer. Blue whales can be found closer inshore, but are often associated with deep coastal canyons, e.g., off central and southern California. The Southern Hemisphere pygmy blue whale (subspecies B. m, brevicauda) has been regarded as restricted to more temperate waters than the “true” blue whale (B. m. intermedia), not often being found much beyond 55°S. The most coastal balaenopterid is the humpback (Megaptera), with long migrations between temper ate/tropical breeding grounds and cold water feeding grounds. In the Southern Hemisphere, much of its journey occurs along the east and west coasts of the three continents. In the Northern Hemisphere, humpbacks are rather more oceanic, but still coastal at some stage in their migration: in the North Pacific they can be found wintering off the Hawaiian Islands and summering off Alaska, and in the western North Atlantic they winter in the Caribbean and summer between New England, the west coast of Greenland, and Iceland.
Minke whales are wide ranging, from polar to tropical waters in both hemispheres. In the Southern Hemisphere the Antarctic species can, with blue whales, be found closest to the ice edge in summer. Elsewhere they can often occur near shore, in bays and inlets. Their migrations are less well defined and predictable than other migratory balaenopterids; in some regions they are present year-round.
The most localized balaenopterid is Bryde’s whale. It is the only species restricted entirely to tropical/warm temperate waters and probably does not undertake long migrations. The two forms—inshore and offshore, in several areas—can differ in their movements. Off South Africa, for example, the inshore form is thought to be present throughout the year, whereas the offshore form appears and disappears seasonally, presumably in association with movements of its food, shoaling fish.
B. Food and Feeding
Although they include the largest living animals, baleen whales feed mainly on very small organisms and are strictly carnivorous, feeding on zooplankton or small fish. In “filter feeding”—sieving the sea—baleen whales are quite different from toothed whales, where the prey is captured individually.
Filter feeding has been described as requiring, in addition to a supply of food in the water, three basic features: a flow of water to bring prey near the mouth; a filter to collect the food but allow water to pass through; and a means of removing the filtered food and conveying it to the stomach for digestion. Baleen whales meet those requirements by (a) seeking out areas where their food concentrates, (b) either swimming open-mouthed through food or gulping it in, (c) possessing a highly efficient filter formed by the baleen plates, and (d) forcing the water containing the food out through die baleen plates and then transferring the trapped food back to the gullet and hence to the stomach. In the latter the tongue is presumed to be involved; in balaenopterids the process is aided by the distensible throat.
While all baleen whales possess a filter based on baleen plates, two rather different systems—essentially “skimming” and “gulping”—have evolved to filter a large volume of water containing food. Each relies on a series of triangular baleen plates, borne transversely on each upper jaw. The inner, longer border (hypotenuse) of each plate bears a fringe of fine hairs, forming a kind of filtering “doormat.” Quite unrelated to teeth (which appear as early rudiments in the gums of fetal baleen whales), baleen is closest in structure to mammalian hair and human fingernails. In the right whales, filtration is achieved with very long and narrow plates in the very large mouth, itself carried in the very large head. The plates, up to 4 m long in bowheads and 2.7 m in other right whales, are accommodated in the mouth by an arched upper jaw and are enclosed in massively enlarged and upwardly bowed lower lips. There is a gap between the rows at the front of the mouth, and the whole arrangement allows the whale to scoop up a great quantity of water while swimming slowly forward. In balaenopterids, with their much smaller heads, the baleen plates are shorter and broader and the rows are continuous at the front. Taking in a large volume of water and food is usually achieved by swimming through a food swarm and gulping, while simultaneously enlarging the capacity of the mouth greatly by extending the ventral grooves and depressing the tongue. The two systems allow, on the one hand, the relatively slow-swimming balaenids to concentrate their rather sparse slow-swimming food over a period, and on the other, the faster-swimming balaenopterids to take in large amounts of their highly concentrated fast-swimming prey over a shorter time.
Typically, baleen whales leed on zooplankton, mainly eu-phausiids or copepods, swarming in polar or subpolar regions in summer. That is particularly so the Southern Hemisphere, where the summer distributions of several balaenopterids depends on the presence of Euphnusia superbn (known to whalers by the Norwegian word “krill”) in huge concentrations in the Antarctic. In the Northern Hemisphere, with a more variable availability of food, balaenopterids are more catholic in their feeding. Humpbacks and fin whales, for example, feeding almost exclusively on krill in the south, commonly take various species of schooling fish in the north.
The variety of organisms taken by the various species in different regions is listed in Table II. While most feeding occurs in colder waters, baleen whales may feed opportunistically elsewhere. All baleen whales but one, the gray whale, feed generally within 100 m of the surface and, consequently, unlike many toothed whales, do not dive very deep or for long periods. Gray whales feed primarily on bottom-living organisms, almost exclusively amphipods, in shallow waters.
The baleen plate structure, particularly the inner fringing hairs, to some extent mirrors the food organisms taken or (in the case of E. superba) different size classes. Thus there is some correlation between decreasing size of prey and fineness of baleen by species, viz. gray, blue, fin, humpback, minke, sei, and right whales. Where food stocks are very dense, e.g., around sub-Antarctic South Georgia, fin, blue, and sei whales may all overlap in their feeding on E. superba.
TABLE II Baleen Whale Food Items
|
|
|
|
Food items |
|
|
Species |
Subspecies |
Common name |
Northern Hemisphere |
Southern Hemisphere |
|
B. mysticetus |
|
Bowhead whale |
Mainly calanoid copepods; euphausiids; |
|
|
|
|
|
occasional mysids. amphipods. |
|
|
|
|
|
isopods, small fish |
|
|
E. glacialis |
|
North Atlantic right |
Calanoid copepods; euphausiids |
|
|
|
|
whale |
|
|
|
E. tuistralis |
|
Southern right whale |
|
Copepods; postlarval Munida |
|
|
|
|
|
gregnria; Enphaiisia snperba |
|
Caperea marginata |
|
Pygmy right whale |
|
Calanoid copepods |
|
E. robustus |
|
Gray whale |
Gaimnarid amphipods; occasional |
|
|
|
|
|
polychaetes |
|
|
M. novaeangliae |
|
Humpback whale |
Schooling fish; euphausiids |
E. snperba (Antarctic); euphausiids. |
|
|
|
|
|
postlarval M. gregaria, occasional |
|
|
|
|
|
fish (ex-Antarctic) |
|
B. acutorostrata B. a. acutorostrata |
N. Atlantic minke |
Schooling fish; euphausiids |
||
|
|
B. a. scammoni |
N. Pacific minke |
Euphausiids; copepods: schooling fish |
|
|
|
B. a. subsp. |
Dwarf ininke |
|
? Euphausiids, schooling fish |
|
B. bonaerensis |
|
Antarctic minke |
|
E. snperba |
|
B. edeni |
|
Bryde’s whale |
Pelagic crustaceans, including |
Schooling fish; euphausiids |
|
|
|
|
euphausiids |
|
|
B. borealis |
|
Sei whale |
Schooling fish |
Copepods, including Calanus; |
|
|
|
|
|
E. snperba |
|
B. physalus |
|
Fin whale |
Schooling fish; squid; euphausiids; |
E. superba (Antarctic); other |
|
|
|
|
copepods |
euphausiids (ex-Antarctic) |
|
B. muscuhis |
B. m, musculus |
Blue whale |
Euphausiids |
|
|
|
B. m. indica |
Great Indian rorqual |
?Euphausiids; copepods |
|
|
|
B. m, intermedia |
“True blue” |
|
E. superba (Antarctic); other |
|
|
|
|
|
euphausiids (ex-Antarctic) |
|
|
B. m, brevicauda |
Pygmy blue |
|
Euphausiids, mainly E. vallentini |
Baleen whale food consumption per day has been calculated as some 1.5-2.0% of body weight, averaged over the year. Given that feeding occurs mainly over about 4 months in the summer in the larger species, the food intake during the feeding season has been calculated at some 4% of body weight per day, ca. 400 kg per day for a large blue whale. To survive the enormous drain of pregnancy and lactation, it has been calculated that a pregnant female baleen whale needs to increase its body weight by up to 65%. The ability to achieve such an increase in only a few months’ feeding indicates the great efficiency of the baleen whales’ feeding system.
C. Predators and Parasites
Apart from humans, the most notable baleen whale predator is the killer whale (Orcinus area). Minke whales have been identified as a major diet item of some killer whales in the Antarctic. Killer whale attacks have been reported on blue, sei, bowhead, and gray whales, although their frequency and success are unknown. Humpbacks often have killer whale tooth marks on their bodies and tail flukes. Humpback and right whale calves in warm coastal waters are susceptible to attack by sharks. There are anecdotal reports of calving ground attacks on humpbacks by false killer whales (Pseudorca crassidens).
A form of harassment, only recently described, occurs on right whales on calving grounds off Peninsula Valdes, Argentina. Kelp gulls have developed the habit of feeding on skin and blubber gouged from adult southern right whales’ backs as they lie at the surface. Large white lesions can result. The whales react adversely to such gull-induced disturbance and calf development may be affected.
External parasites, particularly “whale lice” (cyamid crustaceans) and barnacles (both acorn and stalked) are common on the slower-swimming more coastal baleen whales, such as gray, humpback, and right whales. In the latter, aggregations of light-colored cyamids on warty head callosities have facilitated research using callosity-pattern photographs for individual identification. External parasites are much less common on the faster swimming species, although whale lice have been reported on minke whales (in and around the ventral grooves and umbilicus); the highly modified copepod PeneUa occurs particularly on fin and sei whales in warmer waters. The commensal copepod Balaenophilus unisetus often infests baleen plates in such waters, especially on sei and pygmy blue whales.
A variety of internal parasites have been recorded, although some baleen whales seem less prone to infection than others. They appear, for example, to be less common in blue whales, but prevalent in sei whales. Records include stomach worms (.Anisakis sp), cestodes, kidney nematodes, liver flukes, and acan-thocephalans (“thorny-headed” worms) of the small intestine.
The cold water diatom Cocconeis ceticola often forms a brownish-yellow film on the skin of blue and other baleen whales in the Antarctic. Because the film takes about a month to develop, its extent can be used to judge the length of time an animal has been there. Its presence led to an early common name for the blue whale: “sulfur bottom.”
For many years the origin of small scoop-shaped bites on baleen whale bodies in warmer waters remained a mystery until they were found to be caused by the small “cookie-cutter” shark, Isistius brasiliensis. Some species are highly prone to such attacks. In Southern Hemisphere sei whales die overlapping healing scars can impart a galvanized-iron sheen to the body.
III. Life History A. Behavior
1. Sound Production Unlike toothed whales, baleen whales are not generally believed to use sound for echolocation, although bowheads, for example, are thought to use sound reflected from the undersides of ice floes to navigate through ice fields. However, sound production for communication, for display, establishment of territory, or other behavior, is well developed in the suborder. Blue whales produce the loudest sustained sounds of any living animal. At up to nearly 190 decibels, their long (half-minute or more), very low-frequency (<20 Hz) moans may carry for hundreds of kilometers or more in special conditions. Fin whales produce similarly low (20 Hz) pulsed sounds. Minke whales also produce a variety of loud sounds. Right whales produce long low moans; bowhead sounds, recorded on migration past hydrophone arrays in nearshore leads, have been used in conjunction with sightings to estimate population size off northern Alaska. Southern right whales, at least, seem to use sound to communicate with their calves.
Humpbacks produce the longest, most complex sound sequences in “songs,” described as an array of moans, groans, roars, and sighs to high-pitched squeaks and chirps, lasting 10 or more minutes before repetition, sometimes over hours. It seems that only the adult males sing, generally only in or close to the breeding season. In any one breeding season, all the males sing the same song, changing slightly over successive seasons. Different populations have different songs; so much so, for example, that those off western Australia have a distinctly different song—less complex, less “chirpy”—than that heard on breeding grounds separated by the Australian continent, off the east coast. “Songs” may also be heard in migrating humpbacks, but less so on the cold water feeding grounds, where if they occur at all, they appear generally only as “snatches” or isolated segments.
2. Swimming and Migration With their streamlined bodies, rorquals include the fastest-swimming baleen whales. Sei whales have been recorded at around 35 knots (more than 60 km/lir) in short bursts; minke and fin whales are also known as fast swimmers, the latter up to 20 knots (37 km/hr). Blue whales are among the most powerful swimmers, able to sustain speeds of over 15 knots (28 km/hr) for several hours. On migration, humpbacks and gray whales average about 4 knots (8-9 km/hr) and bowheads only about 2.7 knots (5 km/hr). Migration speeds for southern right whales are not known, but medium range coastal movements off southern Australia indicate 1.5-2.3 knots (2.7-4.2 km/hr) over 24 hrs for cow/calf pairs.
Baleen whales undertake some of the longest migrations known. Gray whales cover 10,000 nautical miles (18,000 km) on the round trip between the Baja California breeding grounds and the Alaskan feeding grounds, among the longest migrations of any mammal. Southern Hemisphere humpbacks may cover as much as 50° of latitude either way between breeding and feeding grounds, a round trip of some 6000 nautical miles (11,000 km). Not all baleen whale migrations are so well marked. The biannual movements of Bering Sea bowheads are governed by the seasonal advance and retreat of sea ice, which varies from year to year. Although Southern Hemisphere blue and fin whales all feed extensively in the Antarctic in summer, the locations of their calving grounds are not known. Sei whale migrations are relatively diffuse and can vary from year to year in response to changing environmental conditions. By comparison, Bryde’s whales hardly migrate at all, presumably being able to satisfy both reproductive and nutritional needs in tropical/warm temperate waters. Even among such migratory animals as humpbacks, it may be that not all animals migrate every year; studies off eastern Australia indicate that a proportion of adult females may not return to the calving grounds each year, and individuals have even been reported in summer farther north. However, Southern Hemisphere migrating humpbacks show segregation in the migrating stream: immatures and females accompanied by yearling calves are in the van of the northward migration, followed by adult males and nonpregnant mature females; pregnant females bring up the rear. A similar pattern occurs on the southward journey, with cow/calf pairs traveling last. Very similar segregation is recorded among migrating gray whales.
Baleen whale migrations have generally been regarded as taking place in response to the need to feed in colder waters and reproduce in warmer waters. Explanations for such long-range movements have included direct benefits to the calf (better able to survive in calm, warm waters), evolutionary “tradition” (a leftover from times when continents were closer together), and the possible ability of some species to supplement their food supply from plankton encountered on migration or on the calving grounds. Corkeron and Connor (1999) have rejected these explanations, suggesting that there may be a major advantage to migrating pregnant female baleen whales in reducing the risk of killer whale predation on newborn calves in low latitudes. They cite in its favor the greater abundance of killer whales in higher latitudes, that their major prey (pinniped seals) is more abundant there, and that killer whales do not seem to follow the migrating animals.
3. Social Activity Large aggregations of baleen whales are generally uncommon. Even on migration, in those species where well-defined migration paths are followed (e.g., gray whales and humpbacks), individual migrating groups are generally small, numbering only a few individuals. It has been stated that predation is a main factor in the formation of large groups of cetaceans, e.g., open ocean dolphins. Given the large size of most adult baleen whales, predation pressure is low and group size is correspondingly small.
Blue whales are usually solitary or in small groups of two to three. Fin whales can be single or in pairs; on feeding grounds they may form larger groupings, up to 100 or more. Similarly, sei whales can be found in large feeding concentrations, but in groups of up to only about six elsewhere. The same is true for minke whales, found in concentrations on the feeding grounds, but singly or in groups of two or three elsewhere. Social behavior has been studied most intensively in coastal humpbacks, e.g., on calving grounds. Male humpbacks compete for access to females by singing and fighting. The songs seem to act as a kind of courtship display. Males congregate near a single adult female, fighting for position. Such aggression can involve lunging at each other with ventral grooves extended, hitting with the tail flukes, raising the head while swimming, fluke and flipper slapping, and releasing streams of bubbles from the blowhole. As a result of such encounters, individuals can be left with raw and bleeding wounds caused by the sharp barnacles. Among southern right whales, similar “interactive” groups are often observed on the coastal calving grounds in winter, involving a tight group with up to seven males pursuing an adult female, but not generally resulting in wounded animals. As for humpbacks, it is not yet certain whether such behavior results in successful mating, although, at least in such right whale groups, intromission is often observed.
Feeding balaenopterids have often been reported as circling on their sides through swarms of plankton or fish. It has been suggested that gray whales feed on their right sides, as those baleen plates are more worn down, presumably through contact with the seabed. The most remarkable behavior, however, is reported from humpbacks. In the Southern Hemisphere, on swarms of krill, they may feed in the same “gulping” way as other balaenopterids. In the Northern Hemisphere, two methods are commonly reported: “lunging” and “bubble netting.” In the former, individuals emerge almost vertically at the surface with their mouths partly open, closing them to force the enclosed water out through the baleen. In the latter, an animal circles below the food swarm; as it swims upward, it exhales a series of bubbles, forming a “net” encircling the prey. It then swims upward through the prey with its mouth open, as in lunging.
B, Growth and Reproduction
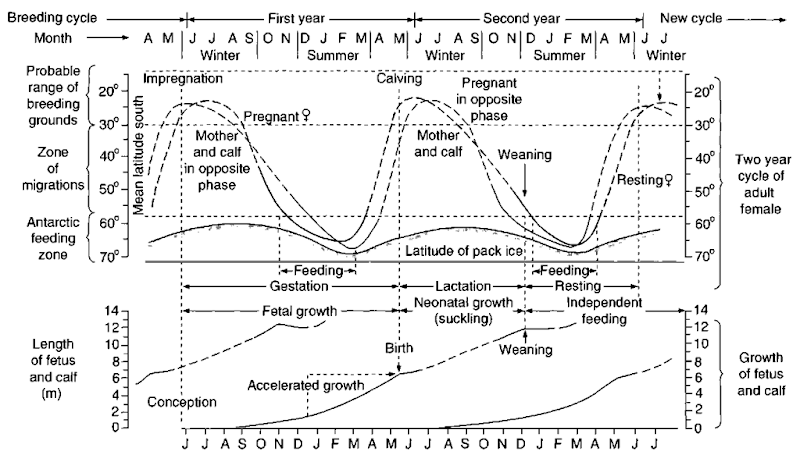
Young baleen whales, particularly the fetus and the calf, grow at an extraordinary rate. In the largest species, the blue whale, fetal weight increases at a rate of some 100 kg/day toward the end of pregnancy. The calfs weight increases at a rate of about 80 kg/day during suckling. During that 7-month period of dependence on the cow’s milk, the blue whale calf will have increased its weight by some 17 tons and increased in length from around 7 to 17 meters. Blue whales attain sexual maturity at between 5 and 10 years, at a length of around 22 meters, and live for possibly 80-90 years. Adult female blue whales give birth every 2-3 years, with pregnancy lasting some 10-11 months.
Other balaenopterids follow the same general pattern (Fig. 3). Mating takes place in warm waters in winter, with birth following some 11 months later. A 7- to 11-month lactation period may be followed by a year “resting,” or almost immediately by another pregnancy. Most adults are able to reproduce from between 5 and 10 years of age and reach maximum growth after 15 or more years. The smallest balanopterid, the common minke whale, is born after a pregnancy of some 10 months, at a length of just under 3 meters. Weaning occurs at just under 6 meters, after 3-6 months. The adult female can become pregnant again immediately following birth, but the resulting short calving interval is generally uncommon in baleen whales: 2-3 years is the norm, although female humpbacks can achieve a similar birth rate, enabling their stocks to recover rapidly after depletion (see Section IV).
Figure 3 “Typical” life cycle of a southern baleen whale.
Right whales follow a similar general pattern, but there are some differences. In right whales, gestation lasts about 11 months and weaning for about another year. Females are able to reproduce successfully from about 8 years (there are records of successful first pregnancies from 6 years), but the calving interval is usually a relatively regular 3 years. For bowheads, it has been reported, rather surprisingly, that while growth is very rapid during the first year of life (from ca. 4.5 meters), it may be followed by a period of several years with little or no growth. Sexual maturity occurs at 13-14 meters; at the reduced growth rate, that would not be reached until 17-20 years. Similarly, evidence shows considerable longevity in this species: stone harpoon heads found in harvested whales and last known to be used off Alaska early this century suggest that individual animals can be at least 100 years old.
IV. Population Status
For centuries, baleen whales have borne the brunt of human greed, for products and profit. Only the sperm whale, largest of the toothed whales, has rivaled them as a whaling target. Black right whales (Eubalaena) were taken in the Bay of Biscay from the 12th century, with the fishery extending across the North Atlantic by the 16th century. Attention then shifted to the Greenland whale (Balaena) near Spitzbergen and later off southern and western Greenland. Both species’ numbers were reduced to only small remnants, and in several areas (e.g., Spitzbergen and Greenland for Balaena and the northeast Atlantic and the North Pacific for Eubalaena) the stocks were virtually exterminated. That destruction was undertaken using the “old” whaling method, with open boats and hand harpoons, on the “right” species—”right” because they were relatively easy to approach, floated when dead, and provided huge quantities of products [oil for lighting, lubrication, and soap and baleen ("whalebone") for articles combining flexibility with strength, such as corset stays, umbrella spokes, and fishing rods].
Development of the harpoon gun and steam catcher, from 1864. increased the rate of catching greatly, but also allowed attention to turn to the largest baleen whales, the blue and fin whales, whose size, speed, and tendency to sink when dead had prevented capture by the old methods. From its beginning in the North Atlantic, then, by the end of the century, in the North Pacific, “modern” whaling’s next and most intensive phase moved south, first in 1904 at South Georgia in the South Atlantic, just within the Antarctic zone. Initially on humpbacks [up to 12,000 were taken in one year (1912), leading to very rapid stock decline] and then on blue and fin whales, southern whaling based on such land stations—in the Antarctic in summer and the tropics in winter—was overtaken from the late 1920s by the great development of pelagic whaling using floating factory ships. Huge annual Southern Hemisphere catches resulted—a maximum of over 40,000 in 1931—averaging around 30,000 animals per year in the later 1930s and again after World War II until 1965. Whereas blue whales had been the preferred target in the 1930s, their great reduction in numbers led to a shift in attention progressively over the years to fin whales, to sei whales in the 1960s, and finally to minke. With depletion of stocks and more stringent conservation measures (killing of humpbacks, blue, and fin whales was banned from the mid-1960s, even though some illegal catching continued until the early 1970s or even later), catches fell to between 10,000 and 15,000 per year in the 1970s. The “old” whaling story had virtually repeated itself—enormous reductions through overfishing of one species or stock leading to exploitation of other species and stocks until, apart from minke whales, only remnants were left. Since 1989, a moratorium on all commercial whaling has eliminated that pressure, with the exception of limited whaling carried our under exemption for scientific research, and, since 1993, limited commercial catching of minke whales in the eastern North Atlantic. Some “aboriginal” whaling has also continued in the Northern Hemisphere, on bowheads off northern Alaska, on gray whales in the Bering Sea, on fin and minke whales off Greenland, and on humpbacks in the Caribbean.
Despite the great scale of the kill in “old” and “modern” whaling, no whale species has become extinct through whaling, although a number of individual stocks have been reduced greatly; at least one, the North Atlantic gray whale, has disappeared within the past 200-300 years. In its most recent (1996) “Red List” of threatened animals (Table 3), the World Conservation Union (IUCN) includes no baleen whale species or stocks as either extinct (EX) or critically threatened (CR) (the latter within the threatened category). Within the threatened categoiy, eight taxa—four species, one subspecies, and three stocks—are listed as endangered (EN); four taxa—one species and three stocks—are vulnerable (VU). Six taxa—two species, one subspecies, and three stocks—are listed as at lower risk (LR), and two taxa—one species and one subspecies—as data deficient (DD).
TABLE III
IUCN Red Lisl Categories for Baleen Whales (1996)
- “Includes E. japonica
- “Okhotsk Sea bowhead whale, Spitzbergen bowhead whale
- ”Hudson Bay bowhead whale, Baffin Bay/Davis Strait bowhead whale
- rBering-Beaufort-Chuckchi Seas bow’head whale
- rfNorth Atlantic and North Pacific northern right whales
- ”Pygmy right whale removed from 1996 Red List
- -^Northwest Pacific gray whale
- ^Northeast Pacific Gray whale
- ”North Atlantic blue whale
- ‘North Pacific blue whale
- “Includes E. japonica
- “Okhotsk Sea bowhead whale, Spitzbergen bowhead whale
- ”Hudson Bay bowhead whale, Baffin Bay/Davis Strait bowhead whale
- rBering-Beaufort-Chuckchi Seas bow’head whale
- rfNorth Atlantic and North Pacific northern right whales
- ”Pygmy right whale removed from 1996 Red List
- -^Northwest Pacific gray whale
- ^Northeast Pacific Gray whale
- ”North Atlantic blue whale
- ‘North Pacific blue whale
Those species under greatest current threat (EN) are the North Atlantic and North Pacific right, the sei, and fin whales, together with the “true” blue subspecies, two of the five bowhead stocks (Okhotsk Sea, Spitzbergen), and the northwest Pacific gray. Next most threatened (VU) are the humpback, two bowhead stocks (Hudson Bay, Baffin Bay/Davis Strait), and the North Atlantic blue. At lower risk (LR) are the southern right and Antarctic minke, one bowhead stock (Bering-Chukchi-Beaufort Seas), the North Atlantic ininke, northeast Pacific gray, and North Pacific blue; all but one are further qualified as conservation dependent (cd, not vulnerable because of specific conservation efforts). The exception is the North Atlantic minke, listed as near threatened (nt, not conservation dependent but almost qualifying as vulnerable). The two taxa for which insufficient information is currently available (DD) are Bryde’s whale and the pygmy blue.
The International Whaling Commission’s Scientific Committee, responsible for the assessments of such stocks’ current status, has reported encouraging recent reversals of stock decline for some stocks of some species. One, the northeast Pacific gray whale, has recovered under protection from commercial whaling (but with aboriginal catches up to some 150 per year) to at or near its “original” (prewhaling) state (ca. 26,000 animals). Similarly, the northwest Atlantic humpback and several Southern Hemisphere humpback populations have been showing marked increases. The latest estimate of the North Atlantic stock, some 10,600 animals in 1993 (cf. 5500 in 1986), must reflect some population growth in the intervening period, whereas two Southern Hemisphere stocks (off eastern and western Australia) have been increasing steadily, at 10% or more per year since the early 1980s. Indeed, in all areas where surveys have been undertaken recently on Southern Hemisphere humpback populations they have been shown to be undergoing some recovery. Three southern right whale stocks (off eastern South America, South Africa, and southern Australia) have been increasing since the late 1970s at around 7-8% per year, although at some 3000, 3000, and 1200 animals, respectively, all are still well below their “original” stock size. Even the “true” blue whale, whose future has been of considerable concern, with estimates for the late 1980s at fewer than 500 animals for the whole Antarctic, has shown recent encouraging signs. Based on a series of Antarctic sightings cruises, mainly for minke whales but including other large whales, the most recent calculations (admittedly using only small absolute numbers sighted) show that the population must have been increasing since the last estimate (1991). As yet the analyses do not permit a firm conclusion on the number now present, although it seems likely to be more than 1000.
The one species or stock for which there is now very great concern is the North Atlantic right whale. At very low absolute abundance (only some 300 animals), not recovering despite protection from whaling in the 1930s, now even decreasing through a reduced survival rate and an increase in calving interval, and subject to increasing removals from ship strikes and fishing gear entanglement, the only way to ensure the species’ survival is to reduce such anthropogenic mortality (ship strike and entanglement) to zero. While research on mortality reduction measures should be pursued, immediate management action is urgently needed.
It has been calculated that the great reduction of baleen whales by whaling, for the Antarctic to around one-third of original numbers and one-sixth in biomass, must have left a large surplus of food—some 150 million tons per year—available for other consumers, such as seals, penguins, and fish. (In a different way, early whaling in the North Atlantic, particularly on right whales, is believed to have influenced the spread of one sea bird—the fulmar—by providing food in the form of discarded whale carcasses.) In response to an increase in available food, there may well have been increases in growth rates, earlier ages at maturity, and higher rates of pregnancy in some baleen whale species. However, the evidence is equivocal, as it is for competition between individual whale species. For some, e.g., right whales and sei, it has been suggested that an increase in one (right whales) could be inhibited by competition with another (sei whales). In the North Pacific, both sei and right whales can feed on the same prey—copepods—and sei whales can at times be “skimming” feeders, like right whales. However, evidence that they actually compete on the same prey, in the same area, at the same time, and even on the same prey patch is lacking. Similarly, there has been much debate and speculation on whether the recovery of the Southern Hemisphere “true” blue whale has been inhibited by an apparent increase in Antarctic minke whales. In that case, there may in fact be very little direct competition for food where the common prey is not limited in abundance (as in the Antarctic) and is available in large patches. The well-authenticated increases in the substantial annual rates for several stocks of Southern Hemisphere humpbacks and right whales and the possibility of at least a limited increase in numbers for the “true” blue whale suggest that such competition is unlikely, at least where, as in the Antarctic, food supplies are abundant.