The interactions of insects with microorganisms range from the cultivation of fungus gardens to intimate associations with bacteria housed within special organs (mycetomes) or dedicated cells (bacteriocytes) of the fat body. Many of these associations have nutritional implications; this article will focus on microbial symbionts that colonize the intestinal tract and are directly involved in digestion.
Although insects produce a wide variety of digestive enzymes, many species harbor an intestinal microbiota that converts a substantial portion of the dietary components to fermentation products before they are resorbed by the intestinal epithelia. The occurrence of such associations is always correlated with a dietary specialization, which indicates that the symbiosis provides metabolic capacities that are normally not available to the host.
Digestive symbioses are most common among insects feeding on wood or other lignified plant materials. The most prominent example is that of wood-feeding termites (Isoptera), which represent the only group of insects whose interactions with intestinal microorganisms has been studied in detail. Here, they serve to illustrate principles that most likely govern also other cases of symbiotic associations in which detailed information on the gut microbiota and its function in digestion is lacking.
BIOCHEMICAL BASIS OF LIGNOCELLULOSE DIGESTION
Why would an insect need help in digesting its food, and which benefits would be gained from sharing this resource with an intestinal microbiota? For xylophagous (wood-feeding) insects, the answers are found in the chemical structure and composition of their fiber-rich, low-nutrient diet.
Structure and Composition of Lignocellulose
The major components of plant cell walls are cellulose and hemicelluloses. Although the glycosidic linkages between the sugar subunits of these structural polysaccharides are relatively easy to hydrolyze, the sheer size of the molecules, their primary and secondary structures, and the intimate physical contact of the different components, especially in lignified cell walls, impart a considerable recalcitrance to enzymatic digestion.
In cellulose, the linear (3-(1—4)-linked polyglucose chains are arranged in microfibrils with a highly ordered, mostly crystalline structure. Cellulose depolymerization is catalyzed by endoglucanases (endo-(3-1,4-glucanases), which cleave randomly within the extremely long polyglucose chains, and by exoglucanases (exo-f3-1,4-ceUobiohy-drolases and (3-glucosidases), which require free nonreducing ends for their catalytic activity. Because native cellulose contains only one nonreducing end per many thousand glucose units, and because the action of endoglucanases is restricted to the amorphous regions of the microfibrils, an efficient cellulolytic system requires the synergis-tic action of both types of enzymes.
Enzymatic activity of cellulases is further impeded by the insolubility of cellulose, and depolymerization is usually the kinetically limiting step even in the digestion of pure cellulose. In the cell wall, the cellulose fibrils are intimately associated with hemicelluloses, which comprise a wide variety of homopolymers and heteropolymers of different sugars and sugar acids. Hemicellulose chains are often branched and lack the highly ordered structure of cellulose. The variety of primary structures requires an equal variety of digestive enzymes, and it is not astonishing that the ability to degrade hemi-celluloses is a prerequisite for an efficient cellulose degradation.
Sound wood is most difficult to digest because, through ligni-fication, the polysaccharides of the secondary plant cell wall are embedded in an amorphous resin of phenolic polymers that provide an efficient barrier to enzymatic attack of the polysaccharides. The lignin macromolecule itself is extremely recalcitrant to degradation since most bonds between the subunits are extremely stable and cannot be hydrolyzed.
Roles of Symbionts in Digestion
For the reasons outlined above, the degradation of plant cell walls requires the synergistic action of many different enzymes and, in the case of lignified substrates, also a mechanism to break up the ligno-cellulose complex. The most efficient cellulose and hemicellulose degraders in nature are microorganisms, i.e., bacteria, protozoa, and fungi. Fungi and certain filamentous bacteria (actinomycetes) are also the only organisms that have developed a strategy for the chemical breakdown of lignin. Not surprisingly, insects and other animals have made use of these capacities by using microorganisms as symbi-onts in the digestion of lignocellulosic food.
In addition to being difficult to degrade, lignocellulose is an extremely nutrient-poor substrate. The C-to-N ratio of sound wood is up to 100-fold higher than that of the insect body. Moreover, a lignocellulosic diet typically lacks most of the essential nutrients required by the animal, such as amino acids, vitamins, and sterols.
In contrast to higher animals, many microorganisms are capable of fixing dinitrogen, assimilating nitrate and ammonia, and synthesizing those amino acids and vitamins essential for the host. Many animals, including insects, have developed means of exploiting these biosynthetic capacities of microorganisms, which include—in the simplest case—the digestion of the intestinal symbionts.
LIGNOCELLULOSE DIGESTION IN TERMITES
The symbiotic digestion of lignocellulose by termites is a complex series of events involving both the host and its gut microbiota, which comprises prokaryotic symbionts (bacteria and archaea) and, at least in lower termites, also protozoa and possibly fungi (yeasts). Although the events in foregut and midgut are mainly the result of host activities, the digestive processes in the hindgut are largely controlled by the symbionts (Fig. 1). Many aspects of lignocellulose digestion are common to all termite species, yet there are several noteworthy differences between the phylogenetically lower and higher taxa.
Host-Related Processes
One of the key contributions of the termite to wood digestion is the action of the mandibles and proventriculus, which grinds
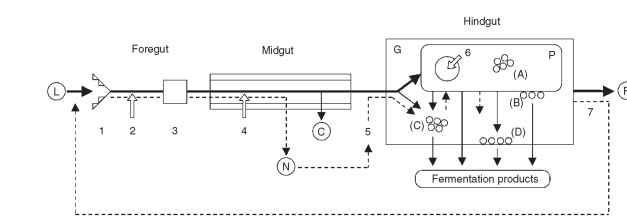
FIGURE 1 Major events in the symbiotic digestion of lignocellulose by wood-feeding lower termites. The bold lines show the path of the insoluble material, the lignin-rich residues of which are released as feces, whereas the thinner lines represent soluble degradation products that are eventually resorbed by the host. The dashed lines indicate the cycling of nitrogenous compounds. Hollow arrows mark the sites where cellulolytic enzymes are secreted. Lowercase letters refer to the different groups of bacteria, which are either endobionts (A) or epibi-onts (B) of the protozoa, suspended in the gut lumen (C), or attached to the gut wall (D). The scheme has been simplified for the sake of clarity; not all possible interactions are shown. Further details are given in the text. L, lignocellulose; C, carbohydrates; N, nitrogenous compounds; F, fecal matter; G, gut lumen; P, protozoa; 1, mandibles; 2, salivary glands; 3, proventriculus; 4, midgut epithelium; 5, Malpighian tubules; 6, phagosomes; 7, proctodeal feeding.
the wood particles down to a microscopic size. This mechanically destroys many lignin-carbohydrate complexes and creates an enormous surface area for the digestive enzymes provided by host and symbionts, thereby relieving much of the kinetic limitations of cellulose digestion. In lower termites, comminution is also a prerequisite for the ingestion (phagocytosis) of the wood particles by symbiotic protozoa.
In all insects, the digesta are exposed to a variety of digestive enzymes secreted by the salivary glands and the midgut epithelium. The persisting dogma that higher animals do not produce cellulases has been unequivocally refuted by the demonstration of endo-glucanase genes in the genomes of termites and many other related insects (Dictyopera). Moreover, all termites investigated express endoglucanases capable of hydrolyzing amorphous cellulose in the cells of the salivary glands or the midgut epithelium (see below). Although systematic studies are lacking, one can safely assume that—as in other insects—most of the easily digestible material has been mobilized and resorbed at the end of the midgut.
Insects lacking a pronounced gut microbiota usually have a short hindgut that serves mainly in recovering water and useful electrolytes from the residual material before the feces are voided. The digestive tract of termites, however, is characterized by one or more proctodeal enlargements and may reach enormous dimensions in length and volume (Fig. 2A). The dilatations increase the residence time of the digesta, thereby prolonging the exposure to the activities of the intestinal microbiota. Moreover, the increased diameter also affects the oxygen status of the “fermentation chambers” because it reduces the proportion of the gut volume rendered oxic by the diffusion of oxygen into the periphery, thus providing a favorable environment for the oxygen-sensitive microbiota and reducing the inevitable loss of fermentation products to aerobic processes (see below).
![Intestinal tracts of insects with fermentation chambers harboring symbiotic microorganisms. (A) Thoracotermes mac-rothorax (Isoptera: Termitidae). (B) Potosia cuprea (Coleoptera: Scarabaeidae). [Reproduced from Werner (1926). Z. Morph. Okol. Tiere. 6, 150-206.] (C) Tipulaflaveolineata (Diptera: Tipulidae).Not drawn to scale. Intestinal tracts of insects with fermentation chambers harboring symbiotic microorganisms. (A) Thoracotermes mac-rothorax (Isoptera: Termitidae). (B) Potosia cuprea (Coleoptera: Scarabaeidae). [Reproduced from Werner (1926). Z. Morph. Okol. Tiere. 6, 150-206.] (C) Tipulaflaveolineata (Diptera: Tipulidae).Not drawn to scale.](http://lh3.ggpht.com/_X6JnoL0U4BY/S8H36UsMJLI/AAAAAAAAYyI/15nBQXc8MHA/tmp5B18_thumb_thumb.jpg?imgmax=800)
FIGURE 2 Intestinal tracts of insects with fermentation chambers harboring symbiotic microorganisms. (A) Thoracotermes mac-rothorax (Isoptera: Termitidae). (B) Potosia cuprea (Coleoptera: Scarabaeidae). [Reproduced from Werner (1926). Z. Morph. Okol. Tiere. 6, 150-206.] (C) Tipulaflaveolineata (Diptera: Tipulidae).Not drawn to scale.
Fiber-Digesting Symbionts
In lower termites, the solid food particles entering the hindgut are immediately phagocytized by the intestinal protozoa (Fig. 3A ).
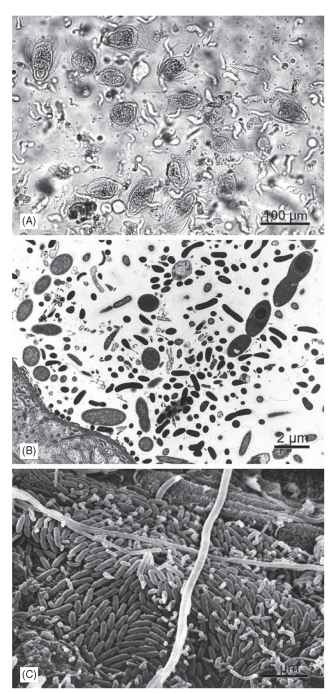
FIGURE 3 Examples of microbial symbionts in the hindgut of Reticulitermes flavipes (Isoptera: Rhinotermitidae), a wood-feeding lower termite. (A) Preparation of anaerobic protozoa from the hindgut of a worker larva, showing the large hypermastigid flagellate Trichonympha agilis, filled with wood particles, and numerous smaller flagellates (mainly oxymonads, Dinenympha spp.). Differential interference contrast photomicrograph taken by U. Stingl. (B) Transverse section through the peripheral hindgut, showing the diverse bacterial microbiota associated with the thin cuticle of the hindgut wall (bottom left). Transmission electron micrograph provided by J. A. Breznak. (C) Preparation of the hindgut wall, showing the dense colonization of the cuticle by numerous rod-shaped and filamentous bacterial morphotypes.
Scanning electron micrograph provided by J. A. Breznak.
These oxygen-sensitive flagellates, which make up a large fraction of the hindgut volume, are essential for wood digestion and represent a major source of cellulolytic and xylanolytic activities in the hind-gut. It appears that the different flagellate species are nutritionally specialized and each species might fill a specific niche in symbiotic digestion. The consecutive action of the host endoglucanases secreted in the salivary glands and the full complement of endog-lucanases and exo-cellobiohydrolases of their eukaryotic symbionts forms the so-called “dual cellulolytic system.”
In phylogenetically higher termites (family Termitidae), the site of secretion of host endoglucanases shifted from the salivary glands to the midgut epithelium. Although all families of lower termites sport a hindgut packed with flagellates, the higher termites contain a largely prokaryotic microbiota. Apparently, other symbionts took over the cellulolytic function of the protozoa in the course of termite evolution. In the case of the fungus-cultivating termites (subfamily Macrotermitinae), which cultivate basidiomycete fungi (Termitomyces spp.) on predigested food in “fungus gardens” located within their nests, the key activities of the symbiotic partner are extensive delignification and conversion of lignocellulose to fungal biomass in the fungus combs. It has been proposed that fungal cellulases ingested together with the comb material are essential for cellulose digestion in the gut of fungus-cultivating termites, but this “acquired enzyme hypothesis” is controversial. Moreover, evidence is accumulating that fungal biomass became an important nutritional basis for certain species.
In all other higher termites cellulolytic activities in the hindgut are probably produced by symbiotic bacteria. Two major bacterial lineages (Fibrobacteres and Spirochetes) seem to be involved in this process. While the former are related to an important group of fiber-degrading bacteria in the cow rumen, the exact role of the latter is still enigmatic.
Noncellulolytic Microbiota
The advent of cultivation-independent methods revealed that the prokaryotic microbiota of termite guts is highly diverse. Most of these symbionts do not take part directly in fiber degradation but seem to play other important roles in digestion and in the nitrogen metabolism of the hindgut. In the lower termites, the majority of prokaryotes is intimately associated with the flagellates, whose large surfaces and biovolume represent an excellent habitat for ectobiotic and endocytobiotic symbionts.
METABOLIC INTERACTIONS
The original concept of the hindgut metabolism in lower termites assumed that the anaerobic flagellates depolymerize the polysaccharides to sugar units, which are then fermented to acetate, hydrogen, and CO2 as the major products. Recent results indicate that the spectrum of fermentation products released by the diverse protozoa is much wider, giving rise to a variety of intermediates that form the substrates of the endobiotic or epibiotic bacteria colonizing the protozoa. Metabolites released by the protozoa or originating directly from the midgut also support numerous bacteria located in the lumen or attached to the wall of the hindgut. All metabolites are eventually converted to a range of short-chain fatty acids (mostly acetate, propionate, butyrate) that accumulate in the hindgut fluid and that are eventually resorbed by the hindgut epithelium (Fig. 1).
Although it is still difficult to assign functions to individual members of the gut microbial community and to localize the microbial populations involved in these reactions, the major roles of different functional groups are beginning to take shape (Fig. 4). At least two
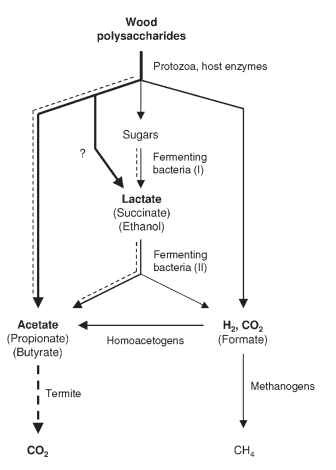
FIGURE 4 Schematic presentation of the metabolic processes involved in the fermentative degradation of wood polysaccharides in the hindgut of lower termites. The dashed lines indicate metabolic fluxes that seem to be strongly influenced by the continuous influx of oxygen into the gut periphery.
metabolically different groups of prokaryotes are involved in the oxidation of the hydrogen or one-carbon compounds produced in the microbial fermentations: (1) methanogenic archaea, which reduce CO2 to methane, and (2) homoacetogenic bacteria, which reduce CO2 to acetate.
Methanogenesis and reductive acetogenesis occur in the hind-gut of all termites, although in wood-feeding species, reductive acetogenesis prevails over methanogenesis as a hydrogen sink and substantially increases the pool of short-chain fatty acids available to the host. Reductive acetogenesis in termite guts is apparently catalyzed by Spirochaetes—a unique feature encountered only in termite hindgut ecosystems. Methanogenesis—as in many other anoxic system the domain of archaea—may amount to a considerable portion of the total respiratory activity in fungus-cultivating and soil-feeding termites. Although methane production is of little use for the host, methane emission acquires global significance owing to the enormous biomass of these groups in tropical rainforests and savannahs.
THE ROLE OF OXYGEN
The steep gradient of oxygen between gut epithelium and gut contents drives a continuous influx of oxygen into the gut. Microsensor measurements have shown that oxygen may penetrate 50-200 |im into the periphery of the hind-gut, leaving only the central portion of the dilated compartments anoxic. The small insect guts have an enormous surface-to-volume ratio, which lends much greater importance to all surface-related processes than in larger animals. Because oxygen removal in the gut periphery is fueled by the fermentative processes in the hind-gut lumen, the maintenance of anoxia is not a trivial issue, and there must be a lower size limit for arthropods with a symbiotic digestion.
The bacteria and protozoa colonizing the gut periphery, especially those directly associated with the gut epithelium (Fig. 3B and 3C), have to be specifically adapted to the presence of oxygen at low levels and may even use oxygen as an electron acceptor. The high fluxes of oxygen into the gut and its efficient removal by anaerobic and microaerobic members of the hindgut microbiota significantly influence the metabolic processes in the hindgut (Fig. 4).
Wood-feeding termites have developed several adaptations
Nitrogen Economy of Termites
that help to compensate for the low nitrogen content of their diet, all of which involve the biochemical capacities of their gut microbiota. The most important strategy is a combination of conservation and recycling and is reminiscent of the application of organic fertilizers in agriculture.
Like in any other insect, uric acid and urea, the waste products of nucleic acid and protein metabolism, are secreted into the digesta via the Malpighian tubules at the midgut-hindgut junction. However, they are not voided with the feces, but are readily mineralized by the hindgut microbiota. The resulting ammonia is assimilated into microbial biomass; it remains to be clarified whether the intestinal protozoa can also assimilate ammonia directly or acquire combined nitrogen by phagocytosis and digestion of other microbial symbionts.
The nitrogen cycle is closed by the digestion of microbial cells. Because termites cannot access the microbes in the hindgut directly, worker larvae solicit hindgut contents from their nestmates. This behavior, which has been termed proctodeal trophallaxis and is unique to this group of social insects, increases in frequency with the level of nitrogen limitation. Digestion of the hindgut contents and resorption of the nitrogenous products probably take place in the foregut and midgut (Fig. 1). The efficiency of nitrogen conservation within the colony is increased further by the consumption of exuviae and dead individuals by nestmates.
While nitrogen recycling creates high ammonia concentrations in the hindgut, which allow the maintenance of an active gut microbiota and thus ensure high rates of carbon mineralization, the low nitrogen content of the food still limits the growth of a termite colony severely. However, the presence of bacteria among the hindgut microbiota capable of fixing atmospheric nitrogen may contribute considerably to increasing the nitrogen pool. It has been estimated that the nitro-genase activity in certain Nasutitermes termites would be sufficient to double the nitrogen content of a colony within a few years, and stable isotope analysis has revealed that 30-60% of the nitrogen in Neotermes koshunensis workers is derived via this pathway.
Although most lower termites are strictly xylophagous, higher termites (Termitidae) show an enormously successful dietary diversification. The fungus-cultivating termites (subfamily Macrotermitinae), which are specialized on degradation of nitrogen-poor plant litter, probably recycle nitrogen within the colony by exploiting the ammonium-assimilating capacities of the fungus. In other subfamilies (Nasutitermitinae, Termitinae, and Apicotermitinae), the diet ranges from sound wood to lignocellulosic plant materials in different stages of humification, including soil and animal dung. Since the C-to-N ratio of the diet decreases with increasing humifica-tion, microbial biomass and nitrogenous components of soil organic matter form a potential source of nutrition. The extreme alkalinity (pH 12) in the anterior hindgut of soil-feeding Termitinae appears to be the key to the exploitation of their nitrogen-rich but recalcitrant diet. During gut passage, alkaline pH and digestive enzymes, probably originating both form the host and the gut microbiota, release fermentable substrates from the ingested humus that are protected from microbial degradation in soil. The transformation and mineralization of nitrogenous soil components in the guts of soil-feeding termites gives rise to high ammonia concentration in their feces and may be an important factor in nitrogen cycling in tropical soils.
DIGESTIVE SYMBIOSES IN OTHER INSECTS
The occurrence of a specific, autochthonous gut microbiota among insects remains to be systematically studied, but sufficient evidence for the presence of a digestive symbiosis has accumulated for representatives of several insect orders.
Phytophagous insects feeding on protein-rich plant material (e.g., caterpillars) usually have a relatively undifferentiated intestinal tract and, because of the rapid gut passage, digest little or no cellulose. Many xylophagous, detritivorous, and humivorous insect larvae, however, possess hindgut dilations that are missing in closely related species with a different feeding habit. The most prominent examples are among the Coleoptera (e.g., Scarabaeidae) and among the Diptera (e.g., Tipulidae) (Fig. 2B and 2C).
Scarabaeids and tipulids have an actively fermenting gut micro-biota, including cellulolytic and hemicellulolytic bacteria and, in the former, also methanogenic archaea, which are attached to brush-like chitinous structures. Also, the guts of omnivorous cockroaches contain a largely prokaryotic microbiota of bacteria and methanogenic archaea, especially when maintained on a fiber-rich diet; many species also harbor anaerobic ciliates with methanogenic endosymbi-onts in their hindguts. Fiber-degrading protozoa, however, which are so prominent among the Isoptera, are found elsewhere only in Cryptocercus punctulatus (Cryptocercidae), which is not astonishing because these wood-feeding cockroaches share a common ancestor with the termites.
Reports on the Orthoptera are somewhat contradictory. The bacteria in the gut of locusts (family Acrididae) have been considered commensals because their absence in germ-free cultures had no obvious effect on the host, although key components of the phe-romone responsible for aggregation of Schistocerca gregaria are produced by its gut microbiota. The situation is different in crickets (Gryllidae), which benefit from the presence of a gut microbiota when raised on a fiber-rich diet. In the hindgut of Acheta domesticus, the density of microorganisms is even higher than that in termites, and there are brush-like supports for the attachment of bacteria that resemble those in scarab beetle larvae.
It is very likely that insects other than termites access protein and recycle nitrogen via digestion by microbial symbionts. Proctodeal feeding is a form of social behavior that is restricted to the termites and the wood-feeding cockroach, C. punctulatus, but theoretically any consumption of feces would also allow access to the microbial protein. A special adaptation to digestion by symbionts seems to be present in Scarabaeidae, in which a reflux of hindgut contents into the alkaline midgut has been observed. Extreme gut alkalinity is encountered also in the midguts of many humivorous, detritivorous, and coprophagous dipteran and coleopteran larvae. The principle of humus digestion in tropical rose chafers (Pachnoda species) bears many similarities to that of soil-feeding termites and may represent a case of convergent evolution.
Freshly hatched insect larvae do not possess a gut microbiota and all instars have to be reinoculated after each molting because hindgut intima and contents are shed in this process. In principle, this is not difficult because many insect larvae eat their exuviae directly after molting, and first instars may pick up their intestinal symbionts together with the food from their environment. However, establishment and maintenance of a specific gut microbiota, as evidenced in the case of termites by many instances of cospeciation between host and symbi-onts, is facilitated by vertical transfer among parent and offspring. In contrast to the symbioses between insects and their intracellular bacteria, this is probably not accomplished by ovarial transmission, but by coprophagy or proctodeal trophallaxis. Since transfer of fresh hindgut content is crucial especially in lower termites because of the oxygen sensitivity of their gut flagellates, the evolution of sociality and symbiotic digestion in termites might have proceeded hand in hand.
MUTUALISTS VS. COMMENSALS
In the symbiosis between lower termites and fiber-digesting flagellates, both partners are indispensable and the mutual advantage is obvious. However, there are many symbionts in the guts of termites and other insects whose presence—to the best of our knowledge— appears to be of no obvious advantage to the host. When the benefit of the association is unidirectional, a symbiont is classified as a commensal, and the host might even benefit from its elimination.
However, it is also not unlikely that in such cases we are merely lacking insight into the symbiont’s role in the symbiosis. An example might be the methanogenic archaea, which are regularly encountered in the hindguts of termites, scarab beetle larvae, and many cockroaches. Although methane, their sole metabolic product, cannot be utilized by the host, methanogenic archaea seem to be an integral part of the gut microbiota. They colonize the hindgut intima or the intestinal protozoa and are often attached to cuticular spines, which apparently represent specific attachment sites for methanogenic symbionts.
Unfortunately, it is often impossible to eliminate a specific member of the intestinal microbial community selectively, and even when possible, it is difficult to distinguish between the direct and the indirect consequences of their elimination. It might also be worth speculating on the problems that would be created by a general absence of specific gut symbionts and on the efforts involved in keeping a gut sterile. Microorganisms are continuously incorporated with the food, and while a rapid passage of the digesta controls microbial growth by washout (as long as attachment is prevented), a slow gut passage (necessary also for the digestion of lignocellulose by host enzymes) would allow the uncontrolled proliferation of foreign microorganisms. Perhaps the promotion of specific symbionts excludes colonization by potential pathogens.
In this context, it is important to realize that the energy expenditure for any microbial symbionts remains relatively small as long as the access of oxygen to the dilated hindgut compartments is limited. A fermentative degradation of carbohydrates to acetate releases only a fraction of the free energy contained in the substrates, which is returned as nutritionally valuable microbial biomass. Together with the added benefit of metabolic properties like nitrogen fixation, ammonia assimilation, and the provision of vitamins, the advantages for the host may be well worth the investment.
CONCLUSION
The digestive symbioses of insects with their intestinal microbiota allow the insects to overcome the severe kinetic limitation of ligno-cellulose digestion and the nutritional restrictions imposed by this
nitrogen-poor diet. Although the situation is somewhat reminiscent of the digestive symbiosis encountered in ruminants, it is important to consider the small size of insects, which affects especially the oxygen status of the gut. From a microbiological point of view, insect guts are not simply anoxic fermentors, but axially and radially structured environments with physicochemically distinct microhabitats. Termites have served as an excellent model for studying symbiotic digestion and the complex interactions within the intestinal micro-bial community. Recently, they are receiving great interest also because of the biotechnological potential of their gut microbiota for the conversion of lignocellulosic wastes to hydrogen and other valuable chemicals. Further efforts targeted at identifying the microbial symbionts and their metabolic capacities in this and other, hitherto understudied, groups of insects are sorely needed.
