Specialized Terms
pattern formation Is the activity by which embryonic cells form ordered spatial arrangements of differentiated tissues; during insect embryogenesis, patterning begins with specification of the anterior-posterior and dorsal-ventral axes, and ends with the formation of the characteristic structures associated with each segment of the head, thorax, and abdomen.
blastomeres The embryonic cells produced during the process of cleavage.
caste Any set of individuals in a colony that are morphologically distinct and/or that perform a specialized task.
complete (holoblastic) cleavage A coordinated series of mitotic divisions wherein the entire volume of the egg cytoplasm is divided into numerous smaller cells that are fully separated from one another and which together comprise a morula.
hemocoel The body cavity of the insect that contains blood and all internal organs.
morphogenesis The phase of embryogenesis in which specific tissues, organs and structures develop.
parasitoid An insect which is free living as an adult but which develops as a parasite in or on the body of another arthropod during its larval stage; parasitoids require only a single host to complete their immature development and almost always kill their hosts; most hosts of parasitoids are other insects, and most parasitoids are in the orders Hymenoptera and Diptera.
polar body The process of forming an oocyte (egg cell) is called oogenesis; at the beginning of oogenesis the oocyte is diploid (called a primary oocyte), but oocytes subsequently undergo two meiotic reduction divisions producing a haploid secondary oocyte that retains most of the cytoplasm and two haploid polar bodies; in most insects polar bodies degenerate soon after fertilization of the haploid oocyte by a sperm; in some endoparasitic Hymenoptera, however, the polar bodies persist and develop into a syncytial membrane that surrounds the embryo; this membrane mediates uptake of nutrients from the host and in polyembryonic wasps participates in partitioning embryonic cells to form additional embryos.
Polyembryony is a form of clonal development in which a single egg produces two or more genetically identical offspring. Many animals are sporadically polyembryonic including humans who occasionally give birth to identical twins. However, a few groups of parasites (some cestodes, trematodes, and insects), colonial aquatic invertebrates (oligochaetes, bryozoans), and mammals (armadillos) are obligately polyembryonic. Among these obli-gately polyembryonic animals, some species of insects produce thousands of offspring per egg. All known species of obligately poly-embryonic insects reside in two orders: Hymenoptera (bees, wasps, and ants) and Strepsiptera. The biology of several polyembryonic wasps has been studied, whereas little is known about polyembry-onic strepsipterans.
Polyembryonic wasps occur in selected genera from four families of Hymenoptera: Braconidae (Macrocentrus), Platygasteridae (Platygaster), Encyrtidae (Copidosoma), and Dryinidae. Polyembryony in these groups evolved from monoembryonic ancestors that produce only a single offspring per egg, because the basal members of these large families are all monoembryonic. The phylogenetic distance between these families also indicates that polyembryony has evolved independently multiple times in the Hymenoptera. Despite their multiple origins, all polyembryonic wasps share the common biology of being endopara-sitoids that lay their eggs into the egg or larval (nymphal) stage of their insect hosts. After oviposition, each egg laid by a polyembryonic wasp develops into a single embryo. This embryo then clonally gives rise to additional embryos that together form an assemblage called a poly-germ or polymorula. Polyembryonic wasps in the family Encyrtidae have evolved a second novelty: a caste system in which morphologically and functionally distinct larvae develop from the same egg. Key factors regulating the development of polyembryonic wasps, caste function and determination, and the evolution of polyembryony are discussed below.
DEVELOPMENT OF COPIDOSOMA AND OTHER POLYEMBRYONIC WASPS
Polyembryonic wasps in the families Platygasteridae and Dryinidae parasitize selected species of Diptera and Hemiptera, respectively, whereas polyembryonic wasps in the families Braconidae and Encyrtidae parasitize Lepidoptera. All polyembryonic encyr-tids oviposit into the egg stage of their host and their progeny complete the larval stage of their development in the host’s final instar. The most studied polyembryonic wasp is the encyrtid Copidosoma floridanum which oviposits into the eggs of different plusiinae moths (Noctuidae) including the common agricultural pest Trichoplusia ni. C. floridanum also produces among the largest broods of any poly-embryonic wasp, with up to 3000 offspring emerging per host.
Development of C. floridanum and other polyembronic wasps consists of three major phases known as early cleavage, proliferation, and morphogenesis (Fig. 1). In C. floridanum, early cleavage begins when the wasp oviposits a tiny, yolkless egg surrounded by a thin chorion into a host egg. The polar nucleus of the egg rapidly separates from the pronucleus which migrates to the posterior pole of the egg along with cytoplasmic germplasm (see below). The C. floridanum egg then initiates first cleavage that results in formation of two equal-sized daughter cells (blastomeres) from cleavage of the pronucleus and an anterior polar cell that contains the polar nucleus. Polar body nuclei are aborted in most insects but they remain viable in polyembryonic encyrtids and form a polar body cell. After first cleavage, all of the germplasm associates with one of the blastomeres. Second cleavage is unequal with the germplasm-containing blastomere dividing into a large and small daughter blastomere with the germplasm always segregating to the smaller cell. The other blastomere in contrast divides into two equal-sized daughter cells (Fig. 1, four-cell stage). Subsequent cleavages are asynchronous resulting in formation of approximately 200 blastomeres of which 4-8 contain germplasm. Concurrently, the nucleus of the polar cell divides without cytokinesis to form a syncytial compartment that migrates as an extraembryonic membrane over the dividing blastomeres. The embryo then emerges from the chorion and continues development in the host body cavity unconstrained by the eggshell. Enveloped by the extraembryonic membrane, this embryo is referred to as the primary morula; it usually implants in the prothorax of the host embryo in proximity to a major tracheal branch or the ventral nerve chord (Fig. 1).
Upon hatching of the host larva, C. floridanum enters the proliferation phase of development (Fig. 1, proliferative phase). This phase begins when the primary morula divides itself into two to five embryonic masses called proliferative morulae that together form a polymorula. Each proliferative morula consists of hundreds of round, seemingly nondifferentiated cells surrounded by the extraembryonic membrane. Each proliferative morula in turn becomes further subdivided by invagination of the extraembryonic membrane resulting ultimately in formation of > 1000 proliferative morulae consisting of 10-30 cells each by the time the host molts to the fourth instar.
The majority of embryos then synchronously enter the third phase of development, morphogenesis, which is first recognizable by the rounded cells in proliferative morulae becoming fibroblastic and compacting. This is rapidly followed by formation of a primordium, gastrulation, and segmentation. Almost all of the morulae that synchronously initiate morphogenesis in the host fourth instar develop into reproductive larvae that eclose in the host’s fifth-instar. These larvae morphologically have a rounded body form and small mandibles (Fig. 1, reproductive morphogenesis). Reproductive larvae rapidly consume the hemolymph and internal organs of the host, pupate within the remaining exoskeleton of the host, and subsequently emerge as adult wasps. In contrast, some proliferating morulae, initiate morphogenesis during the host’s first through fourth instar and develop into the second caste, soldier larvae, that are morphologically recognized by their elongate body form and larger mandibles (Fig. 1, soldier morphogenesis). On average about 4% of the total number of larvae produced per host by C. floridanum are soldiers, although certain conditions result in up to 25% of larvae developing into soldiers. Soldier larvae never molt and die from desiccation after their reproductive-caste siblings consume the host. As discussed below, soldiers are also functionally distinct from reproductive larvae.
Limited comparative studies of other polyembryonic encyrtids indicate they too produce soldier and reproductive larvae that appear to develop similarly to C. floridanum. Studies of selected polyem-bryonic braconids, platygasterids, and dryinids indicate they too lay small, yolkless eggs that undergo complete cleavage to form a single embryo, followed by a proliferation stage and morphogenesis. Unlike encyrtids, however, polyembryonic wasps in these other families lack a caste system and only produce larvae that consume the host pupate and emerge as adult wasps.
CASTE DETERMINATION
Caste formation in social insects like ants, bees, or termites is usually a phenotypically plastic trait whereby each individual has the capacity to develop into one caste or another. Caste fate is usually mediated by the interaction between environmental factors (photoperiod, crowding, pheromones, nutrition) and endocrine physiology during the larval or nymphal stage that commit individuals to developing into one caste or

FIGURE 1 Development of the polyembryonic wasp Copidosomafloridanum in its host Trichoplusia ni. At the top of the figure is shown a wasp ovipositing into a host egg. Major host stages are illustrated on the left with duration of each stage indicated in days. The host egg hatches in 3 days followed by development of the host larva through five instars from day 3 to 15. For wasp stages, the newly laid C. florida-num egg undergoes early cleavage and formation of a primary morula during the host’s egg stage. The proliferation stage then occurs during the host’s first-fourth instar. Some embryos undergo morphogenesis during this period and develop into soldier larvae, whereas the majority of embryos synchronously initiate morphogenesis at the end of the fourth instar and develop into reproductive larvae that consume the host at the end of its fifth-instar. The polar-body derived extraembryonic membrane is shown in gray, the germ line derived from the small blast-omere at the four-cell stage of early cleavage is shown in red, and presumptive somatic stem cells are shown in blue.
another as an adult. Caste formation is also associated with differential expression of a number of genes associated with metabolic functions. Polyembryonic wasps obviously differ from most caste-forming insects in that both castes arise from a single egg that develops in a seemingly identical environment (the host). Although environmental cues from the host could play a role in caste fate, several studies collectively suggest extrinsic factors from the host are not the key factors that regulate caste formation. Instead, caste fate appears to be intrinsically regulated
by the asymmetric parceling of the germ line during the proliferation stage of embryogenesis. In most animals including insects, germline stem cells (GSCs) generate either more of themselves or differentiate into gametes which give rise to offspring. GSCs derive from primordial germ cells (PGCs) that are determined during embryogenesis. Phylogenetically advanced insects like Drosophila establish the germ line at the beginning of embryogenesis by inheritance of maternal determinants supplied in the form of specialized germplasm.
These maternal determinants also prepattern the embryonic axes of Drosophila. C. floridanum and other polyembryonic wasps are relatively closely related to Drosophila, yet the dramatic differences in early development (see above) make it unclear whether these insects determine the germ line similarly. Using a conserved marker for germ cells in insects and many other animals called vasa , studies with C. floridanum indicate that germplasm is prepackaged into eggs and is inherited specifically by the small blastomere that forms after second cleavage (see above) (Fig. 1). This asymmetry persists in the primary morula and during the proliferation phase suggesting that daughter cells of the small blastomere all inherit Vasa protein and thus represent a PGC lineage. Most proliferating morulae inherit two to eight cells that express Vasa protein, but a smaller proportion of proliferating morulae inherit no Vasa expressing cells (Fig. 1). Upon initiation of morphogenesis, embryos that contain Vasa expressing cells always develop into the reproductive caste. During morphogenesis, these Vasa—expressing cells also localize to the posterior of the embryo and give rise to the gonads (Fig. 2). In contrast, embryos lacking Vasa expressing cells always undergo morphogenesis into soldiers (Fig. 2). Evidence that presence and absence of the germ line is responsible for caste fate derives from manipulative experiments in which elimination of the small Vasa-containing blastomere at the four-cell stage of embryogenesis results in formation of only soldier larvae. Thus, in addition to specifying the germ line, PGCs in C. floridanum also have noncell autonomous functions that regulate caste fate. Loss of the PGC lineage at the four-cell stage also dramatically reduces the number of embryos produced suggesting an additional role in regulating proliferation. In contrast, the fact that soldier larvae develop when maternal germ determinants are eliminated suggests these factors are not required for pre-patterning of embryonic axes as found in other insects.
CASTE FUNCTION
The evolution of specialized castes has occurred most often in species that live in groups comprised of closely related individuals, and that occupy resource-rich but defensible resources. These conditions exist in well-known eusocial insects like ants and termites as well as in less studied caste-forming insects like gallmaking thrips and aphids. These conditions also exist in polyembryonic wasps that propagate clonally inside the nutrient-rich but defensible environment of hosts. In Copidosoma sp., reproductive larvae are so named because they are the only offspring that develop into adult wasps with reproductive functions. On the other hand, hosts parasitized by these wasps are also commonly attacked by other parasitoids that then compete for the same host resources. Cruz reported that one function of soldier larvae is to recognize and kill heterospecific competitors by attacking them with their mandibles. Field studies with C. floridanum, further reveal that risks from intraspecific competitors may be equal or even greater. Commensurate with this risk, C. floridanum soldiers exhibit a well-developed ability to discriminate kin from non-kin, and aggressively attack non-relatives (Fig. 2). In both hetero- and intraspecific defense, soldier larvae increase their own fitness by assuring the survival of their reproductive siblings. Because selection acts at both the level of the individual and brood, the ratio of investment in reproductive and soldier larvae would also be predicted to vary depending on how large or small the threat from competitors might be. Such adaptive phenotypic plasticity occurs in C. floridanum where the proportion of embryos developing into soldiers increases greatly in hosts attacked by competitors.
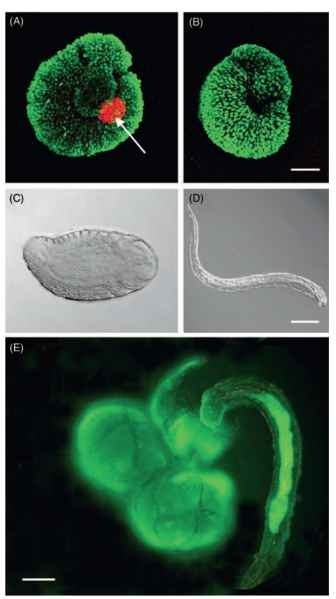
FIGURE 2 Caste determination and function in C.floridanum. (A) Lateral view of a reproductive-caste embryo during morphogenesis at the onset of visible segmentation. The embryo remains coiled with the anterior and posterior extremities of the germ band oriented to the right. Germ cells expressing Vasa protein (red) are clustered in the posterior of the embryo in a location corresponding to the future gonad (arrow). Embryonic nuclei are counter stained with H1 histone (green). (B) Lateral view of a soldier caste embryo lacking germ cells. Scale bar in (B) equals 25 |im. (C) Newly eclosed reproductive larva with head oriented to the right and abdomen to the left. (D) Newly eclosed soldier larva with same orientation. Scale bar in (D) equals 50 |im. (E) Soldier larva attacking embryos from a nonrelative wasp. Embryos were labeled with a vital dye (green) while the gut of the soldier is labeled green from consuming cells of the foreign embryos. Scale bar in (E) equals 75 |im.
A second, more complex function of soldiers in at least some species of polyembryonic encyrtids is the killing of male siblings. Like most Hymenoptera, all caste-forming polyembryonic wasps are haplodiploid with male offspring developing from unfertilized eggs and female offspring developing from fertilized eggs. In C. flori-danum, female wasps usually lay two eggs per host (one male and one female) and as a consequence most broods are of mixed sex. However, soldier larvae develop almost exclusively from female eggs resulting in soldiers that are fully related to their sisters but not to their brothers. These female soldiers attack and kill most brother embryos but fully refrain from attacking sister embryos; resulting in a strongly female-biased sex ratio (>95%) for the adult wasps that emerge from hosts. The asymmetric production of female soldiers that kill brothers but not sisters likely arose as a consequence of genetic conflict among siblings. Under conditions of limited host resources, daughters favor a more female-biased sex ratio than the mother and sons because of higher relatedness to clonal sisters. Sons in contrast favor a less biased sex ratio than mothers or sisters, because of higher relatedness to clonal brothers. Increasing opportunities for males to obtain mates away from the host from which they emerge further increases conflict. The biased production of aggressive soldiers by females, therefore, appears to resolve this conflict in favor of daughters. Selective pressure for large brood size may also be less for brothers than sisters, because a few males almost always survive in mixed-sex broods which mate a large number of sisters before dispersing and seeking additional mates that emerge from other hosts.
THE EVOLUTION OF POLYEMBRYONY
Given that polyembryony occurs in several lineages of parasitic Hymenoptera and Strepsiptera, but is unknown from other insect taxa, suggests factors associated with a parasitic lifestyle are likely key to the evolution of this unusual form of development. Most hymenopter-ans are in the suborder Apocrita. Most free-living apocritans (pollen-nectar feeders and predators) are restricted to the Aculeata (Chrysidoidea, Vespoidea, and Apoidea), whereas the other major apocritan superfamilies are comprised almost exclusively of parasi-toids. Parasitoids exhibit two developmental strategies. Many develop as ectoparasitoids that lay their eggs externally on hosts and feed as larvae by rasping a hole through the host’s cuticle, while others develop as endoparasitoids that inject their eggs into the hemocoel of the host where the larvae feed on blood or tissues. Phylogenetic analyses indicate that all aprocritans likely evolved from an ectoparasitic ancestor related to contemporary orussoids. Thereafter, endopara-sitism has evolved independently at least eight times from different ectoparasitic ancestors. Although polyembryony itself has evolved multiple times, all polyembryonic wasps and strepsipterans are themselves endoparasitic as are their nearest monoembryonic ancestors.
The association between endoparasitism and the evolution of poly-embryony is unlikely coincidental. Free-living insects like Drosophila, ants, or honey bees develop in a terrestrial environment independent of the parent. Adaptations for survival include a thick chorion to protect the embryo, and an abundant yolk source to supply nutrients for development. Ectoparasitoids confront the same environmental conditions, but endoparasitoids develop in the nutrient-rich environment of another insect where protection from desiccation and prepackaging of a yolk source are no longer required. Unconstrained by the need for a prepackaged source of nutrition (yolk) and a rigid chorion, the embryos of endoparasitoids also have the potential to evolve alterations in early development as well as to increase significantly in volume and biomass during embryogenesis. If endoparasitism is an important prerequisite for the evolution of polyembryony, the loss of yolk and alterations in early development associated with polyembryonic wasp eggs should also occur in at least some lineages of monoembryonic endoparasitoids. This in fact is seen in several different families of endoparasitic Hymenoptera that produce tiny, yolk-free eggs that undergo early cleavage to produce a single embryo similar to the early phases of embryogenesis exhibited by polyembryonic species. Early cellularization by endoparasitic wasp embryos allows for spatial and temporal uncoupling of processes like germ line specification and embryo axis polarity that typically occur concurrently in Drosophila and other terrestrial insects. Thus, within the nutrient-rich environment provided by a host, alterations in early development combined with the evolution of cell-autonomous activity by germ cells allowed for the evolution of a novel proliferation stage during embryogenesis in which differentiation of presumptive somatic stem cells is inhibited. Thereafter, selective pressure from hetero- and/ or intraspecific competitors has likely favored a second novelty: a caste system founded on asymmetric parceling of the germ line that results in development of altruistic, sterile soldiers that protect their still proliferating reproductive-caste siblings. Additional variables such as host distribution and the mating structure of populations could then further select for different patterns of progeny allocation and sex-specific differences in soldier production.
