Stoneflies comprise a hemimetabolous order of16 families and more than 2000 species of aquatic insects distributed on all continents except Antarctica, and most major islands except notably Cuba, Fiji, Hawaii, and New Caledonia. They are primarily associated with running water, where nymphs inhabit mineral or organic substrates of streambeds, and the winged adults rest throughout their seasonal lives in streamside microhabitats such as rocks, moss, debris, leafpacks, and riparian vegetation. A few species occur in waveswept substrates of cold alpine and boreal lakes, or in intermittent streams. Stonefly adults (Fig. 1D and 1E) are variable in size from about 5 to 50 mm, and in color from black to green or yellow, often marked with distinctive light or dark patterns. The aquatic adult of one species known from the depths of Lake Tahoe (Capnia lacustra) and a few other species are apterous (wingless), but most adults are winged. The wings of males and females of some species, or particular populations of a species, are shortened (brachypterous) and they do not fly, but the typical condition is of two pairs of wings as long or longer than the abdomen (macropterous). As the ordinal name (Plecoptera = folded wing) describes, the hind wings typically have an expanded posterior (anal) lobe that folds longitudinally under the main wing (Fig. 1D).
Stoneflies are relatively slow, somewhat awkward fliers that typically fly short distances to disperse, to search for mates, or, for females to deposit eggs.
Adults ( Fig. 1D) have ten abdominal segments; the genitalia of males are distinctive at the generic and species levels, and consist mainly of various external manifestations of the ninth and tenth
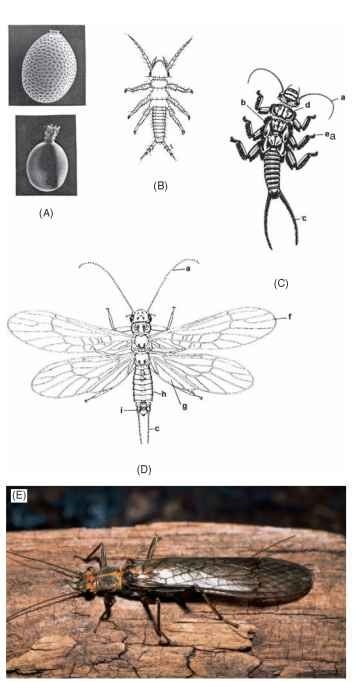
FIGURE 1 Life stages of stoneflies. (A) Eggs. (B) Hatchling (first instar). (C) Late instar. (D) Adult male. a, antennae; b, gills; c, cerci; d, prothorax; e, tarsal claws; f, forewing; g, hind wing anal area; h, abdomen; i, epiproct of male genitalia; (E) Adult of the stonefly Calineuria californica (Banks).
segments such as paired hooks, lobes (paraprocts), or sclerotized stylets and in some taxa a median probe (epiproct) of various shapes. During compulation, the copulatory organ (aedeagus) normally inside the abdominal cavity is everted ventrally from the genital opening on the ninth sternum. The external female genitalia consists of a flap-like subgenital plate covering the genital opening on the eighth abdominal sternum, a structure that the male grasps or holds with his hooks or lobes during copulation. The wings, 3-segmented tarsus, genitalia, and a pair of usually multi-segmented tails (cerci), arising from the 10th abdominal segment, generally characterize stonefly adults. Nymphs (or larvae) (Fig. 1B and 1C) may or may not generally resemble their adults. They are gill-less or have diagnostic simple or branched tracheal gills arising from different parts of the body such as near mouthparts, ventral head, thorax, coxae or abdomen, and they always have multi-segmented tails (cerci). Stubs or the basal remnants of gills are retained as vestigial structures in the adults of some taxa, and aid in their identification to family and genus. The long, multi-segmented nymphal cerci become reduced to fewer segments in some adults, or to a single segment in adult males of the families Leuctridae, Nemouridae, and some Taeniopterygidae.
TAXONOMY AND GENERAL DISTRIBUTION
The Plecoptera is divided into the two suborders Arctoperlaria and Antarctoperlaria. The Arctoperlaria are distributed in the Northern Hemisphere, except the family Notonemouridae which occurs only in southern South America, Southern Africa, Madagascar, Australia, Tasmania, and New Zealand, and some genera of Perlidae such as Anacroneuria and Neoperla that have moved south across the equator in recent times, perhaps from 15 to 30 mya. The Arctoperlaria is further divided into the Group Euholognatha (containing six families: Capniidae, Leuctridae, Nemouridae, Notonemouridae, Taeniopterygidae, and Scopuridae) and the Group Systellognatha (containing six families: Chloroperlidae, Peltoperlidae, Perlidae, Perlodidae, Pteronarcyidae, and Styloperlidae. The Euholognatha have mouthparts adapted for herbivory (scrapers, grazers, collector-gatherers, shredders, gougers, and detritivores), including molariform mandibles, and its species occur with few exceptions in streams of various sizes. The Systellognatha, except Peltoperlidae and Pteronarcyidae whose mouthparts are similar to those of Euholognatha because of convergent evolution to herbivorous food habit, have mouthparts mainly adapted for predation, including sharp-cusped mandibles and toothed lacinia for grasping and holding prey. The systellognathan families Perlidae and Peltoperlidae have very few species in arctic and subarctic streams.
The suborder Antarctoperlaria, as the name implies, is restricted in distribution to the Southern Hemisphere. In some areas, recently invading genera of Arctoperlaria, such as Anacroneuria (Perlidae) in South America and Neoperla (Perlidae) in Africa, have outcom-peted them. The suborder contains four families; Austroperlidae, Diamphipnoidae, Eustheniidae, and Gripopterygidae. The Austroperlidae and Gripopterygidae live in a wide variety of habitats and the Diamphipnoidae and Eustheniidae are restricted to relict populations in the southern Neotropical and Australian regions. Each of the 16 families of Plecoptera has unique combinations of wing venational, genital, gill, and other characteristics.
ECOLOGICAL IMPORTANCE
Stoneflies are integral and important food web components of most stream ecosystems throughout the world and therefore are almost exclusively beneficial insects. The various taxa have radiated to utilize virtually every type of food and substrate habitat resource available to them. The nymphs are variously detritivores, herbivores, insectivores, or omnivores, and in some species the diets of nymphs shift from detritovory or herbivory, through omnivory, to strict insec-tivory as development proceeds. In turn, they become food for larger insectivores and fishes, and are therefore important in the energy dynamics of stream food webs. Particular taxa are usually associated with particular stream microhabitats, such as the interspaces of loose gravel or cobble substrates, leafpacks, detritus, debris, or logs.
The nymphs of numerous species have evolved to coexist in a relatively harmonious, noncompetitive way in given stream ecosystems by partitioning their food, space, and time resources. Most stonefly species require relatively undisturbed conditions of the streams they historically inhabit and therefore are important biological indicators of stream water quality. They constitute the “P” component of one of the major biomonitoring indexes of “clean water species,” called the EPT Index (“E” for Ephemeroptera; “T” for Trichoptera), used for assessing water quality and degree of stream disturbance by humans.
LIFE HISTORY AND BEHAVIORS
Adults
Adult stoneflies (Fig. 1D and 1E) usually emerge during the night from nymphs that have crawled out of the water onto objects projecting from streams or on the stream bank, such as entrained leafpacks, logs or debris, rocks, or riparian vegetation. Some species of Euholognatha are black and emerge under ice or snow cover in winter. A light colored (teneral), clumped-winged, soft adult emerges from the last instar skin during the molt through a split in the head and dorsal thoracic segments. Males of many species emerge a day or more before females, so that they are present and searching when females appear. After some degree of hardening, both sexes become cryptic, hiding in crevices or vegetation during inactive periods, usually during the day, and becoming active in mate finding and other activities typically at night. Adults of Systellognatha have reduced mouthparts and typically do not feed, but may ingest liquids or nectar. Adults of Euholognatha feed variously on algae, lichens, flower pollen and nectar, or soft fruits. For most species of stoneflies, the details of transformation, dispersal, feeding, inactivity periods, and longevity are unknown. Generally, adults live only one to a few weeks, and are mostly actively engaged during that time in the reproductive activities of mate finding, copulation, and oviposition.
Communication, Mate Finding, and Mating
The primary method of communication for locating mates in the Northern Hemisphere stonefly suborder Arctoperlaria is vibrational signaling through substrates. The vibrations are produced by tapping or rubbing the abdomen on the substrate or by body tremu-lations transferred to the substrate. The signals of most insects that use this method consist of simple volleys of evenly spaced vibrations, but stoneflies have evolved a much more diverse and complex system of vibrational communication than is known for other insect groups. The currently accepted paradigm of how this behavior, generally known as drumming, evolved suggests that the ancestral method of signal production was percussion, and that signals were monophasic volleys of evenly spaced drumbeats. Natural selection favored increasingly complex signals, leading to greater efficiency of communication and mate finding among species, and possibly increased capability for sexual selection to measure reproductive fitness. An increasing complexity of particularly male call signals may have evolved through the following three behaviors: (1) more sophisticated signaling methods, sometimes associated with specialized co-evolving ventral abdominal structures, (2) rhythmic patterning of signals, and (3) possible use of selected natural substrates for signal transmission. The result has been that current species signal variously by percussion, scraping, or rubbing the abdomen on the substrate (abdominal-s ubstrate stridulation) or tremulation (vibrations produced by push-ups or rocking motions of the body without abdominal contact with the substrate). Signal rhythms are species-specific and vary from evenly or unevenly spaced monophasic volleys to variously spaced diphasic or grouped signals.
The entire mating system of stoneflies involves communication as well as aggregation and movement behaviors of both sexes, the actual copulation, and post-mating behaviors. The typical system in Arctoperlaria involves the following complex of behaviors: (1) initial aggregation of sexes at encounter sites near streams, (2) calling by males with species-specific signals during ranging search, (3) duet establishment by virgin females answering the male call if effective vibrational communication distance from her is achieved, (4) a localized search by the male in a “triangulation” or other pattern for the now stationary female while both continue dueting, and (5) almost immediate mating after the male locates and contacts the female. Males are polygamous and presumably continue calling and searching during their short reproductive lives.
Typically, mated and unguarded females reject subsequent male advances by raising and curving their abdomens. Southern Hemisphere stoneflies of the suborder Antarctoperlaria have never been documented to drum; therefore, their communication-search system for mate finding is unknown, but they may have evolved a highly specific encounter site aggregation behavior that enables sufficient mate-locating ability without vibrational or other forms of intersexual communication.
Mating in stoneflies involves the male mounting the female, curving his abdomen around her left or right side and engaging the sub-genital plate, pulling it down with his external genitalia. This effectively matches her genital opening beneath the plate with a dorsal position between his cerci where the aedeagus will project. His aedeagus is everted from beneath the ninth sternum and expands backward and upward between his cerci into the female. Sperm are usually conveyed into the female by this intromittent aedeagus, but in some species sperm are conveyed through a hollow male epiproct or are externally deposited onto the female opening to be subsequently aspirated into the bursa (vagina) by telescoping movements of her abdomen.
Eggs and Oviposition
The eggs of stoneflies (Fig. 1A) vary considerably in size, shape, and details of chorionic (egg shell) ornamentation and sculpturing. Commonly, eggs are spindle-shaped, but they also may be spherical, flattened or three-sided. Frequently, an anterior collar is present and the shell surface may be smooth or ornamented with ridges or the hexagonal pattern of impressions formed by the cells lining the ovarian chambers where the eggs are produced. Micropyles (sperm entrance holes) penetrate the chorion completely, and may have associated surface grooves or ornate projections that serve as sperm guides. Actual penetration and fertilization by sperm of the egg is, as in most insects, delayed until the eggs are being stored or passed through the oviduct just prior to oviposition. The sperm are stored in the female spermathecum between copulation and fertilization. There also may be shallow pores leading to elaborate respiratory networks within the chorion. Eggs have sticky membranous or gelatinous surface coverings, and sometimes filament-like projections with hooked tips, that swell and help the eggs attach to substrates under water close to the selectively optimal sites where females deposit them.
Eggs are deposited by females in pellets or masses, each containing numerous eggs, that the females hold on the subgenital plate. They release the egg masses either directly into the water by splashing into the surface during an oviposition flight, or by contacting shallow water while running near the shore, or by dropping eggs from the air while flying over water. Females of some Capniidae are also known to completely submerge themselves and crawl along the bottom and scrape the egg mass off onto the substrate.
In most species, embryonic development proceeds directly and is complete within 3-4 weeks. In other species, particularly those adapted to intermittent streams or streams subjected to extremes in temperature, embryonic development may be arrested for from 3 months to 1 or more years, and hatching is thus delayed until environmental conditions are favorable for nymphal survival. Such arrested development is termed diapause, and it may be manifested in both egg and nymph stages.
Nymphs
Hatchlings (Fig. 1B) emerge from the egg by pushing on the cho-rion with the front of their head. The shell breaks into two halves, or splits leaving a hinged cap and opening through which the first instar crawls out. The first instars are unpigmented with few body hairs, have fewer than 12 antennal and 6 cercal segments, and gills and wingpads are absent, reduced, or represented only by knobs or stubs. Little is known about the food, habitat, or behavior of hatchling and early instar stonefly nymphs. The few species that have been studied feed mainly in detritus or the microflora-fauna on the surface of decomposing leaves.
Nymphs progressively develop and grow through about 10-24 sizes (instars). Full development of particular species requires from 4 months to 3 to 4 years. During this time there is a molt between each instar, addition of antennal and cercal segments, usually addition of body hairs, a progressive increase in size of wingpads, and appearance and development of gills (if present in particular taxa) and characteristic pigment patterns. Growth of a particular species may be sustained at an even pace or seasonal, with fast and slow stages. In temperate climates growth is generally rapid during spring and fall and slowed or arrested (diapause) during extreme temperatures in summer or winter. But, interestingly, a number of euholognathan species, particularly in the families Capniidae and Taeniopterygidae, have adapted to experience their major growth in late fall and winter and emerge as adults during winter on ice or snow or in early spring during ice breakup. Completion of development and subsequent emergence as adults in temperate climates, therefore, may occur during any season, depending on altitude, latitude, and species.
Stoneflies have diversified their food habits such that different species fill about every conceivable major food niche in steams. Some species are herbivore-detritivores throughout their development, some are insectivores throughout development, and some experience an ontogenic (developmental) shift from herbivory-detritivory through omnivory and finally to carnivory. Characteristics of mandibles give a clue to food habits. The mandibles of herbivores have molariform surfaces or scraping ridges and those of carnivores sharp teeth for grasping and tearing. The food of carnivores species consists primarily of the other aquatic insects of their communities such as midge larvae (Chironomidae), mayfly nymphs (Ephemeroptera), caddisfly larvae (Trichoptera), and occasionally the smaller nymphs of other stoneflies. Nymphs may be opportunists, or in some cases are very selective for the size, behavior, and taxa of their prey. Prey are captured, grasped by the head with the lacinia and mandibles, and usually swallowed whole, head-first.
Particular species of nymphs are found in certain types or sizes of streams at particular latitudes or elevations and often in specific micro-habitats. Rare and endemic species have very specific biological and physical requirements and therefore continue to exist only in pristine or little-disturbed sections of streams that in many instances are now found only in remote areas or in, or adjacent to, protected national parks or preserves. Many species have broader requirements and are more widespread or ubiquitous over large areas of continents in a wide variety of habitats. Only a few species are tolerant of the conditions of streams disturbed by siltation, alteration of natural temperature regimes or chemical pollution. Stoneflies depend on substrates as a place in which to live. Slender species live in the interspaces of gravel, cobble, or vegetable debris such as leaf packs. Partitioning of microhabitats, and consequent microdistribution, is characteristic of most stonefly assemblages in a given stream. Most species live in the surface layers of a streambed, but a few live deep in loose mineral substrates such as glacial till, and sometimes in the water-filled spaces of such substrates for considerable distances deep and lateral from the margins of the surface stream. Nymphs are sometimes found drifting in the water column of streams. This results from being dislodged by some physical disturbance or entering the water column as a behavioral means of dispersal, using the flow of water. Drifting enables nymphs to escape predators or move to a less populated habitat where food and/or space resources are more available or of higher quality.
Life Cycles
With few exceptions, a full generation of the egg, nymph, and adult stages of a stonefly species requires 1-4 years. For 1-year (uni-voltine) cycles the nymphal growth portion may be “fast,” requiring only 4-7 months, or “slow,” requiring nearly a full year. The fast type is characteristic of species that diapause for variable times up to 8 months during warm or dry periods of streams; the slow type characterizes species whose nymphal stage requires about 11 months. Species requiring more than 1 year for a generation are termed sem-ivoltine. Those that live in intermittent streams may diapause in the egg stage during drought periods for more than a year, then have a fast-growing nymph for only 4-6 months. Semivoltine species living in cold streams may have a short egg stage, with nymphs requiring 2 or more years to develop, or a year-long diapausing egg stage, with nymphs requiring 1-3 years to develop. The life cycles and resource requirements of stoneflies are important considerations for developing stream management strategies.
