Specialized Terms
idiobiont A life style in which a parasitoid paralyzes and/or arrests the development of a host at oviposition, providing its larvae with an immobilized static resource on which to feed.
koinobiont A life style in which a parasitoid allows its host to continue to feed and/or develop after oviposition, such that its larvae feed on an active host that is only killed at a later stage.
synovigenic A form of reproduction in which an adult female continues to produce and to mature eggs throughout adult life.
encapsulation The response of host blood cells, known as plasmatocytes, to the presence of a parasitoid egg or larvae which results in the formation of a multilayered capsule that causes the death of the parasitoid through asphyxiation.
Parasitoids are insects with a parasitic larval stage that develops by feeding on the body of a single host insect or other arthropod. Feeding by the larval parasitoid invariably results in the death of its host, and the resulting adult parasitoid is a free-living insect. Thus parasitoids occupy an intermediate position between predators and true parasites; in contrast to parasites they kill their host like a predator, but in contrast to predators they require only a single host to complete their development as is the case for parasites.
Nearly 10% of described insect species are parasitoids and, as they often belong to poorly known groups of insects, it has been suggested that they are more likely to represent 20-25% of all insect species. Since the first accounts of insect parasitism at the beginning of the 18th century, parasitoids have attracted considerable attention because of their potential to regulate the abundance of insect hosts in both natural and managed ecosystems. Numerous insect pests have been effectively controlled through releases of insect par-asitoids in biological control programs around the world. Although not always applicable or successful, biological control continues to provide some dramatic examples of sustained long-term control of invasive insect pests. As a result of the research conducted through biological control programs, parasitoids have also become important model organisms for the study of ecology, behavior, and evolution.
ORIGIN AND DIVERSITY OF PARASITISM
About 77% of the known parasitoid species belong to the parasitic Hymenoptera, an arbitrary division of the suborder Apocrita of the order Hymenoptera. It is generally believed that parasitism evolved just once in the Hymenoptera, and that the Apocrita together with the sawfly family Orussidae form a holophyletic group that includes all of the known parasitic wasps. Although the larvae of some species may initially feed on microorganisms in the tunnels of wood-boring insects, the young larvae of others feed externally and subsequently internally on larvae of the borers themselves. Many horntails and wood wasps (sawfly superfamily Siricoidea) carry symbiotic fungi in pouches located at the base of the ovipositor (mycangia) that are inoculated at oviposition, allowing the larvae to feed on infected and partially digested wood. However, not all siricoids carry fungal sym-bionts and some may have evolved to kill those that did, and subsequently feed on the more nutritious dead insect rather than the wood. This trait is seen among the present day orussids.
Within the parasitic Hymenoptera there are more than 66,000 described species of parasitoids in 10 superfamilies that are, with rare exception, exclusively parasitic (Table I). However, parasitoids are also known from five other insect orders. In contrast to the Hymenoptera, parasitism has evolved repeatedly within these orders, either from a fungal feeding (e.g., Rhipiphoridae), dead organism feeding (e.g., Phoridae, Sarcophagidae), predatory (e.g., Carabidae, dipteran families) or phytophagous (e.g., Lepidoptera) ancestor. For example, parasitism is estimated to have occurred 17 times in the Diptera producing over 17,000 described parasitoid species, and 14 times in the Coleoptera producing a further 2450 parasitoid species (Table I). Parasitism is unusual and rare among the remaining three orders.
PARASITOID LIFE STYLES
Parasitoids are frequently categorized as having larvae that are either ectoparasitic (external feeders) or endoparasitic (internal feeders), and either solitary (one per host) or gregarious (several per host) in their development. However, a more useful categorization of the life styles of parasitoids is the dichotomy between idiobiosis and koinobiosis. Idiobionts paralyze and/or arrest the development of a host at oviposition, providing their larvae with an immobilized static resource on which to feed. In contrast, koinobionts allow the host to continue to feed and/or develop after oviposition, such that their larvae feed on an active host that is only killed at a later stage. Although ecto- and endoparasitism are distinct modes of parasitism, the separation of idiobiont and koinobiont life styles is less clear. There should be no problem in correctly categorizing parasitoids that attack an active stage of the host life cycle (larva or adult) or those that allow a transition between life-cycle stages of the parasitized host (egg-prepupal and pupal-adult parasitoids). However, the distinction is not always so obvious for parasitoids that attack and complete their development in an inactive stage of the life cycle (egg or pupa).
Idiobionts
Idiobiont parasitoids frequently attack hosts that are concealed in plant tissues or exposed hosts that provide some other form of physical protection, such as the scale covering of armored scale insects. Thus, many have long ovipositors to reach their concealed hosts, and strong mandibles to escape from concealed locations. The
Table I |
||
| The Main Superfamilies of Hymenoptera and Families of | ||
| Other Insect Orders That Contain Parasitoids, with | ||
| an Indication of Either the Total Described Species for Taxa | ||
| That are Exclusively Parasitic, or the Number of Described | ||
| Parasitoid Species for Taxa (Followed by*) that Include | ||
| Many Nonparasitic Species | ||
| Order | Supeifamily/family | World species |
| Hymenoptera | Orussidae | 75 |
| Trigonalidae | 90 | |
| Stephanidae | 300 | |
| Megalyridae | 45 | |
| Mymarommatidae | 10 | |
| Evanioidea | 1,100 | |
| Cynipoidea | 2,335* | |
| Chalcidoidea | 22,000* | |
| Proctotrupoidea | 2,135 | |
| Platygastroidea | 4,000 | |
| Ceraphronoidea | 800 | |
| Ichneumonoidea | 25,000 | |
| Chrysidoidea | 3,000* | |
| Vespoidea | 5,500* | |
| Diptera | Acroceridae | 520 |
| Bombylidae | 4,500 | |
| Nemestrinidae | 300 | |
| Phoridae | 600* | |
| Pipunculidae | 1,380 | |
| Conopidae | 800 | |
| Sarcophagidae | 100* | |
| Tachinidae | 9,200 | |
| Strepsiptera | Stylopida | 580 |
| Mengenillidae | 10 | |
| Coleoptera | Carabidae | 1,600* |
| Staphylinidae | 400* | |
| Rhipiphoridae | 450 | |
| Lepidoptera | Pyralidae | 1* |
| Epipyropidae | 40 | |
| Neuroptera | Mantispidae | 15* |
| Trichoptera | 1* | |
majority of idiobionts that attack concealed hosts have larvae that are ectoparasitic and feed on hosts in the later stages of their development. An ovipositing adult first injects venom into the host, to induce temporary or permanent paralysis, and then oviposits on or near to the immobilized host. In a few cases (some Bethylidae, Braconidae, and Eulophidae in the Hymenoptera), the parent female remains with the parasitized host, either to defend her young offspring against competitors or hyperparasitoids, or to ensure continued paralysis of the host. Ectoparasitic idiobionts are often long lived, feeding for somatic maintenance on honeydew, nectar, or other plant exudates. They are synovigenic, meaning that they continue to mature eggs throughout adult life, and produce large yolk-rich eggs using nutrients gained from host feeding (see below). Consequently, ectoparasitic idiobionts have a relatively low rate of host attack and a low fecundity. The hatching larvae are protected from desiccation by the host concealment, but develop continuously and rapidly to consume the host before it is attacked by scavengers or microbial decay.
Endoparasitic egg and pupal parasitoids are also considered to be idiobionts, although as noted above, this categorization is less clear. In this case the hosts are frequently exposed rather than concealed, and in place of paralysis, development of the host appears to be arrested by secretions either from the ovipositing adult (egg parasi-toids) or from the young larva (pupal parasitoids). Endoparasitism of host eggs is facilitated by the lack of an immune defense, but it is not known how pupal idiobionts avoid such defenses in their hosts.
The absence of an intimate association between the juvenile stages of an ectoparasitic idiobiont and its host allows this group of parasitoids to have a broader host range, using a variety of hosts that share a common habitat or host plant. Some endoparasitic idiobionts are more restricted in their host range, however, perhaps because of a need for more specific cues in host recognition, or for detoxification of the chemical defenses of exposed hosts.
Koinobionts
All dipteran parasitoids, the majority of coleopteran parasitoids, and many hymenopteran parasitoids adopt a koinobiont way of life. Koinobiont parasitoids attack both exposed and concealed hosts, and the majority of species have endoparasitic larvae. Endoparasitic koinobionts attack a broad range of developmental stages of their hosts. Access to concealed hosts is facilitated either by attacking the more accessible egg or young larval stage of their host (hymenop-teran koinobionts only) or by production of a free-living first-instar larva that can complete the location of a suitable host.
Koinobionts typically oviposit into the body of their hosts with minimal disruption to the normal activity of the host. The hemocytic immune response of the host can result in the encapsulation of para-sitoid eggs and larvae, and is a significant obstacle to endoparasitism. This response can be overcome by (1) avoidance by placing eggs in host tissues such as the salivary gland or nerve ganglia; (2) evasion by producing an egg with a fibrous coat or a coating of proteins that are not recognized as foreign to the host; (3) suppression by injecting immu-nosuppressive polydnaviruses or virus-like particles at oviposition; or (4) subversion by allowing host hemocytes to form a sheath around the developing larva that is attached to the host tracheal system to avoid asphyxiation. Koinobiont are also mostly synovigenic, but differ from idiobionts in having a much greater fecundity with the majority of their eggs produced early in adult life. Koinobiont eggs are relatively small and enrichment occurs after oviposition through absorption of nutrients from the host hemolymph. The greater emphasis on early reproduction is often associated with a shorter adult life, although longevity is greatly influenced by access to a sugar food source.
Many koinobionts exhibit protracted larval development, remaining as a first-instar larva within the host until the latter has retreated to its pupation site. This both allows the host larva to complete its development without suffering any debilitating effects from parasitism, and permits the parasitoid to remain in its most competitive stage, as first-instar larvae bear strong defensive mandibles, until the host has gained maximum size as a resource for parasitoid larval development. Thus, koinobiont parasitoids are often more specialized in their host range than idiobionts, because of their more intimate relationship with an actively feeding or reproducing life stage of the host and the need to overcome its immune defenses.
BEHAVIOR AND INTERACTIONS
Host Feeding
Many idiobiont adult females acquire nutrients from host by feeding on the body fluids that exude from wounds inflicted by the ovipositor, a process known as host feeding. Some small gregarious parasitoids are able to host feed on the same host individuals used for oviposition, but in most cases host feeding is destructive and can be responsible for substantial levels of host mortality. Destructive host-feeders select smaller host individuals for host feeding because they are unsuitable for parasitism, and then use larger hosts for oviposition.
Interactions among Parasitoids
As the majority of host insects are attacked by several different parasitoid species, a variety of trophic and competitive interactions occur among them. Hyperparasitism is a trophic interaction that occurs when a secondary parasitoid parasitizes a primary parasitoid (see section on Hyperparasitism). Competitive interactions include superparasitism and multiparasitism. Superparasitism occurs with more than one oviposition by one or more individuals of the same parasitoid species into the same host individual. The resulting intraspecific competition between parasitoid larvae leads to the death of all but one individual in the case of solitary parasitoids, and to male bias in the sex ratio and reduced adult size among the progeny of gregarious parasitoids. Multiparasitism is the corresponding interspecific competition that results from oviposition by two or more different parasitoid species in the same host individual. The outcome of multiparasitism is often indeterminate; it can favor the species that attacked first but it can also be fixed with a strong competitor being the victor whatever the sequence of attack. A particularly interesting form of the latter is cleptoparasitism, in which the success of host location by a cleptoparasitoid is facilitated by its response to chemical markers used by an inferior competitor to avoid reattacking a previously parasitized host. The cleptoparasitoid is able to steal the host from its inferior competitor by having an aggressive first-instar larva that is able to kill the original occupant of the host.
Clutch Size and Sex Ratio
One of the most important “decisions” for a gregarious parasitoid is how many eggs to lay on a particular host. Clutch size increases with the size and quality of a host, but decreases as the rate of host encounters increases and often decreases with the age of the para-sitoid. Gregarious parasitoids adjust clutch size to match the quality and frequency of hosts encountered, thereby balancing the need to maximize reproductive output and to minimize intraspecific competition among the larval brood. As hymenopteran parasitoids use haplodiploid reproduction (males develop from unfertilized hap-loid eggs, females from fertilized diploid eggs), parent females can choose the sex of their offspring. Solitary parasitoids tend to allocate male eggs to small or low quality hosts and female eggs to large or high-quality hosts, but typically produce a balanced sex ratio. In contrast, female bias is frequent in the sex ratio of gregarious parasitoid broods where the probability of sibling mating is high and sons compete within broods for mates. Thus, local mate competition within broods of gregarious hymenopteran parasitoids leads to the allocation of just enough sons to be able to mate effectively with all the daughters in the brood.
PARASITOID COMMUNITIES
Parasitoids can utilize hosts in a variety of ways to support the development of their progeny. For example, a lepidopteran host such as the gypsy moth (Lymantria dispar) is attacked at different stages through its life cycle by a series of 13 species in 6 parasitoid guilds
(Fig. 1). Very few terrestrial insects escape the attention of parasitoids, exceptions being the few taxa that are either too small (e.g., Adelgidae) or too well defended (e.g., Dactylopius mealybugs). However, the parasitoid load, or number of parasitoid species supported by a host species, varies tremendously. For example, the average number of parasitoid species associated with an aphid is 1.7 in comparison with 12.4 for bivoltine Lepidoptera. In contrast, aquatic insects support relatively few parasitoids and are most vulnerable during the nonaquatic stages of their life cycle. It is notable, however, that a few mostly very small parasitoids are known to be truly aquatic using their legs or wings to swim through the water to locate submerged host eggs.
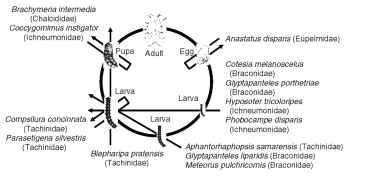
FIGURE 1 The parasitoid assemblage associated with the gypsy moth (Lymantria dispar) as it passes through its life cycle in Eurasia. Arrows indicate the host stages attached and killed by the six different guilds of parasitoid species.
Parasitoid load is determined by a combination of phylogeny, feeding niche, abundance, and chemical defense. The absence of a pupal stage greatly reduces the parasitoid load of a host, but even among the holometabolous insects, beetles consistently support far fewer parasitoids than moths, indicating the importance of host phy-logeny. The feeding niche of a larval host also has an important influence on parasitoid load, being greatest amongst those hosts that have restricted mobility but poor protection (e.g., leafminers, casebear-ers) and reduced either by greater mobility (e.g., external feeders) or by greater protection (e.g., borers). The greater the abundance of a host, the greater its parasitoid load, as a number of less specialized parasitoids and even incidental species are able to make use of an abundant resource. Then, finally, the sequestration of defensive plant chemicals by externally feeding host larvae appears to offer a further line of defense that can lead to a reduction in parasitoid load.
It has frequently been suggested that parasitoid diversity declines from temperate zones to the tropics. Such a pattern is evident for the very species rich superfamily, the Ichneumonoidea (Ichneumonidae and Braconidae), but is not upheld among the Chalcidoidea. Nonetheless, the decline, or absence of an increase, in overall parasi-toid diversity in the tropics is an interesting anomaly in comparison to the increase in diversity of their insect hosts in the tropics. An increased level of predation (notably by ants) in the tropics, a general reduction in the abundance of each host species due to fragmentation of resources, and a greater availability of plant-based chemical defenses for host insects to use for protection may all help to account for this anomaly.

