Insecticide resistance is an example of a dynamic evolutionary process in which chance mutations conferring protection against insecticides are selected in pest populations. This article reviews the origins and mechanisms of resistance, the factors that influence its severity, and the current options for combating its detrimental impact on agricultural productivity and human health.
INTRODUCTION
The genetic variation inherent in all populations is the consequence of random mutations within individuals, their recombination through meiosis, and the dispersal of genes between populations (gene flow). This variation is then shaped by the chance events of genetic drift and by the deterministic process of natural selection. The latter phenomenon eliminates alleles (gene variants) that reduce the fitness of an organism and preserves those that are neutral or that increase fitness. In eukaryotes, the phenotypic changes (adaptations) that result from this process are seldom visible over a human lifetime. The development of pesticide resistance by arthropods, however, is a spectacular exception to the rule.
Since the 1940s, synthetic insecticides have been used on an increasing scale to control the insects, mites and ticks that cause immense crop losses and pose major threats to public and animal health. However, because many of the target species have evolved resistance, some of these chemical control programs are failing. At the current time, more than 500 arthropod species have evolved resistance to at least one insecticide class, and a few populations of some of those species are now resistant to all, or almost all, of the available products (Fig. 1).
The evolution of insecticide resistance is an obvious constraint on effective pest management, both of agricultural pests and of vectors of disease. Resistance in Anopheline mosquitoes threatens the sustainability and efficacy of indoor residual spraying and insecticide-treated bednets for malaria control, and annual crop losses in the United States alone, related to insecticide resistance in pests, have been estimated at $1.4 billion. In order to compensate for such reduced control, farmers or spray operators are often driven to increase the concentrations of insecticides used and/or make repeated treatments to the same areas. This strategy, however, simply increases selection for further resistance development. The resulting “pesticide treadmill” in which ever greater quantities of insecticide are used to overcome burgeoning resistance problems undoubtedly contributes to our seeming inability to use insecticides
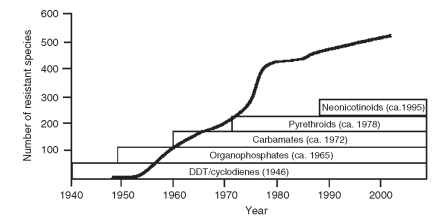
FIGURE 1 DDT was the first insecticide to be used on a global scale. The first case of DDT resistance was recorded in 1946, signaling the beginning of a continuing war of attrition. Over the next 60 years, the number of resistant arthropod species increased rapidly in response to the development and use of insecticides. Horizontal bars denote time over which particular insecticide groups have been used, and dates are the year in which resistance was first documented [Adapted with permission from Georghiou, G. P. (1990). Overview of insecticide resistance. In "Managing Resistance to Agrochemicals" (M. D. Green, H. M. Le Baron, and W. K. Moberg, eds.), pp. 18-14. ACS Symposium Series 421.
judiciously, despite an increasing awareness of their detrimental effects on human health and the environment. Despite numerous initiatives aimed at reducing pesticide use, the acreage over which the developed world applies insecticides has remained stable for over a decade. The United Kingdom alone treats approximately 6 million hectares of land with insecticides each year (reflecting multiple applications to the same areas).
It is proving impossible to combat resistance by simply embarking on a chemical arms race in which redundant and resisted insecticides are replaced by others. The development of a new insecticide takes a decade and costs approximately $180 million. There are consequently few products that are deemed by agrochemical companies to be worth supporting all the way to the market place. Moreover, for those chemicals that are registered, resistance often arises within a few years (and sometimes within a few seasons).
DIAGNOSIS OF RESISTANCE
Although a large number of laboratory bioassay methods have been developed for detecting and characterizing resistance, most of these are limited to defining phenotypes and provide little or no information on the underlying genes or mechanisms. Thus, although bioassays remain the indispensable mainstay of most large-scale resistance monitoring programs, much attention is being paid to developing more incisive techniques that not only offer greater precision and turnover rates but also diagnose the type of mechanism(s) present and, whenever possible, the genotypes of resistant insects. A variety of approaches are being adopted for this purpose, including electrophoretic or immunological detection of resistance-causing enzymes, kinetic and end-point assays for quantifying the activity of enzymes or their inhibition by insecticides, and DNA-based diagnostics for mutant resistance alleles. An increased understanding of the insect genome has, in the past decade, allowed researchers to design microarrays (sets of cloned DNA molecules) that contain large numbers of coding regions associated with resistance. These microarrays can be used to probe insects for the incidence and expression of resistance alleles and are a powerful tool for the incrimination and diagnosis of resistance mechanisms.
The sensitivity of some of these techniques is exemplified by work on the green peach aphid, Myzus persicae. In northern Europe, this insect possesses three coexisting resistance mechanisms: an overproduced carboxylesterase conferring resistance to organophosphates, a modified acetylcholinesterase (MACE) conferring resistance to certain carbamates, and target-site resistance [i.e., knockdown resistance (kdr)] to pyrethroids. It is now possible to diagnose all three mechanisms in individual aphids by using an immunoassay for the overproduced esterase and molecular diagnostics for the MACE and kdr alleles. The combined use of these techniques against field populations provides up-to-date information on the incidence of the mechanisms, serves to inform growers of potential control problems, and assists with developing optimal strategies for the management of M. persicae.
EXTENT OF RESISTANCE
In some insects, resistance extends only to a few closely related compounds in a single chemical class. It may be very weak or restricted to a small part of the insects’ geographical range. At the other extreme, some populations of widespread pests, such as the yellow fever mosquito (Aedes aegypti), the diamondback moth (Plutella xylostella), the Colorado potato beetle (Leptinotarsa decem-lineata), and the sweet potato whitefly (Bemisia tabaci” now resist most or all of the insecticides available for their control.
The most extensively used insecticide classes are the organochlo-rines, organophosphates, carbamates, and pyrethroids. These four insecticide classes accounted for 70% of all insecticide use globally in the year 2000 and have generally been the most seriously compromised by resistance. Many principles relating to the origin and evolution of resistance can be illustrated solely by referring to these fast-acting neurotoxins. In recent years, however, there has also been a worrying increase in resistance to more novel insecticides. These include compounds attacking the developmental pathways of arthropods (e.g., chitin synthesis inhibitors), their respiratory processes [e.g., mitochondrial electron transport inhibiting (METI) acari-cides], their digestive systems [e.g., Bacillus thuringiensis (Bt) endotoxins], as well as newer neurotoxins (e.g., neonicotinoids, spinosyns, and indoxacarb). Neonicotinoids were introduced in the mid-1990s, and are currently the fastest growing class of insecticides worldwide. Although resistance has been slow to develop, mutations that render their target site (the nicotinic acetylcholine receptor) less susceptible and enzymes conferring enhanced detoxification are now being reported in an increasing range of pests.
ORIGINS AND BREADTH OF RESISTANCE
Insecticides are not mutagenic at their field application rates and are not the causative agents of insecticide resistance. Rather they act to select favorable mutations inherent in the population to which they are applied. Some attempts to estimate the rates at which resistant mutations occur have been made. The treatment of blowflies ( Lucilia cuprina) with a chemical mutagen resulted in the production of a dieldrin-resistant target-site mutation in less than one per million individuals, and less empirical measures of mutation rates suggest an underlying figure of anywhere between 10~3 and 10~16, undoubtedly dependent on the resistance mechanism involved.
Some studies have found the incidence of resistant mutations to be worryingly high. A recessive allele conferring resistance to Bt toxins in unselected populations of the tobacco budworm, Heliothis virescens, was estimated to be present in about one in every thousand individuals in some areas of North America. Sixteen in every hundred insects (a frequency of 0.16) were found to carry a Bt-resistant recessive allele in unselected populations of the pink bollworm, Pectinophora gossypiella, in Arizona cotton fields. Mutations conferring resistance to some Bt toxins seem common, even in species that have never been exposed. Frequencies of 0.003 are reported for a recessive allele conferring resistance to the Cry3A toxin in the beetle Chrysomela tremulae and to Cry2A in unexposed Helicoverpa armigera populations. Despite this, Bt cotton (engineered to express Bt toxins) remains effective in the control of pest species, suggesting that such estimates need to be interpreted carefully. A recent study on ancient museum specimens of the sheep blowfly from Australia showed that a mutated esterase that confers resistance to the orga-nophosphate malathion was already common in the population long before the introduction of this insecticide.
Resistant mutations seldom confer protection to just a single toxin. Most commonly, they exhibit differing levels of resistance to a range of related and unrelated insecticides. In its strictest sense, the term cross-resistance refers to the ability of a single mechanism to confer resistance to several insecticides simultaneously. A more complex situation is that of multiple resistance, reflecting the coexistence of two or more resistance mechanisms, each with its own specific cross-resistance characteristics. Disentangling cross-resistance from multiple resistance, even at the phenotypic level, is one of the most challenging aspects of resistance research.
Cross-resistance patterns are inherently difficult to predict in advance because mechanisms based on both increased detoxification and altered target sites can differ substantially in their specificity. The most commonly encountered patterns of cross-resistance tend to be limited to compounds in the same chemical class. However, even these patterns can be very idiosyncratic. For example, organo-phosphate resistance based on increased detoxification or target-site alteration can be broad ranging across this group or highly specific to a few chemicals with particular structural similarities. The breadth of target-site resistance to pyrethroids in houseflies is also dependent on the resistance allele present. The kdr allele itself affects almost all compounds in this class to a similar extent (~10-fold resistance), whereas resistance due to the more potent super-kdr allele is highly dependent on the alcohol component of pyrethroid molecules, ranging from about 10-fold to virtual immunity. Cross-resistance between insecticide classes is even harder to anticipate, especially for broad-spectrum detoxification systems whose specificity depends not on insecticides having the same mode of action but on the occurrence of common structural features that bind with detoxifying enzymes.
Empirical approaches for distinguishing between cross-resistance and multiple resistance include repeated backcrossing of resistant populations to fully susceptible ones, to establish whether resistance to two chemicals cosegregates consistently, and reciprocal selection experiments, whereby populations selected for resistance to one chemical are examined for a correlated change in response to another. If available, biochemical or molecular diagnostics for specific resistance genes can assist considerably with tracking the outcome of genetic crosses or with assigning cross-resistance patterns to particular mechanisms.
MECHANISMS OF RESISTANCE AND THEIR HOMOLOGY
Depending on the mechanism involved, resistance has been shown to arise through structural alterations of genes encoding target-site proteins or detoxifying enzymes, or through processes affecting gene expression (e.g., amplification or altered transcription). Examples of the former include the following.
• Enhanced metabolism of insecticides by cytochrome P450 monoxy-genases can potentially confer resistance to most chemical classes. Much of the evidence for this mechanism in the past was indirect, based on the ability of monoxygenase inhibitors to reduce the magnitude of resistance when used in combination with insecticides in bioassays. More recently, information from genome sequences such as that of the fruit fly Drosophila has been used to probe for differences in the expression of individual members of large gene families such as P450s.
• Enhanced activity of glutathione S-transferases (GSTs) is considered to be potentially important in resistance to some classes of insecticide, including organophosphates. Like monoxygenases, GSTs exist in numerous molecular forms with distinct properties, making the identification of enzymes associated with resistance very challenging.
• Enhanced hydrolysis or sequestration by esterases (e.g., carboxy-lesterases) capable of binding to and cleaving ester bonds undoubtedly plays an important role in resistance to organophosphates and pyrethroids. Biochemically, this is the best-characterized detoxification mechanism. Sometimes (e.g., for mosquitoes, blowflies, and M. persicae), the esterases have been identified and sequenced at the molecular level. Resistance caused by increased esterase activity can arise through a qualitative change
in an enzyme, improving its hydrolytic capacity, or (as in mosquitoes and aphids) a quantitative change in the titer of a particular enzyme that already exists in susceptible insects.
The following examples appear to show that although some adaptations to the environment are unpredictable (e.g., the modifications of the forelimbs for flight are very different in birds, bats, and pterodactyls), the opportunities for insects to modify or reduce binding of insecticides, hence to develop target-site-based resistance mechanisms, are very limited indeed. It is likely that most of the mutations capable of conferring resistance do not allow the organism to retain normal functioning of the nervous system, and are therefore lethal and cannot be selected.
• Pyrethroids act primarily by binding to and blocking the voltage-gated sodium channel of nerve membranes. Knockdown resistance, or insensitivity of this target site, is now unequivocally attributed to structural modifications in a sodium channel protein. The same amino acid substitution (leucine 1014 to phenylalanine) in a sodium channel protein confers a “basal” kdr phenotype in a range of species including house flies, cockroaches, the green peach aphid, the diamondback moth, and a mosquito (Anopheles gambiae). This phenotype may subsequently be enhanced (to “super-kdr” resistance) by further mutations that also recur between species.
• y-aminobutyric acid (GABA) receptors are targets for several insecticide classes including cyclodienes (a subclass of the organo-chlorines), avermectins, and fipronils. The primary mechanism of resistance to cyclodienes and fipronils involves modification of a particular GABA receptor subunit, resulting in substantial target-site insensitivity to these insecticides. The target-site mechanism of cyclodiene resistance has been attributed to the same amino acid substitution (alanine 302 to serine) in the GABA receptors of several species of diverse taxonomic origin including Drosophila, several beetles, a mosquito (A. aegypti), a whitefly (B. tabaci), and a cockroach (Blattella germanica) . When susceptible individuals of the sheep blowfly (L. cuprina) were exposed to the mutagen ethyl methanesulfonate (EMS), and their progeny screened for resistance to dieldrin (a cyclodiene), surviving insects exhibited an alanine-to-serine amino acid substitution in the GABA receptor identical to that found in nature.
• Organophosphates and carbamates exert their toxicity by inhibiting the enzyme acetylcholinesterase (AChE), thereby impairing the transmission of nerve impulses across cholinergic synapses. Mutant forms of AChE showing reduced inhibition by these insecticides have been demonstrated in several insect and mite species. Biochemical and molecular analyses of insecticide-insensitive AChE have shown that pests may possess several different mutant forms of this enzyme with contrasting insensitivity profiles, thereby conferring distinct patterns of resistance to these two insecticide classes. Some of these resistance mechanisms are illustrated schematically in Fig. 2 .
SPREAD OF RESISTANCE GENES
The recurrence of specific resistance mutations within and between taxa begs another question: have such mutations arisen repeatedly within the same species, or have they appeared on a limited number of occasions and subsequently spread through migration and/or human agency? Although there is molecular evidence for some resistance genes having several independent origins in the same species (e.g., for target-site resistance to cyclodienes in the red
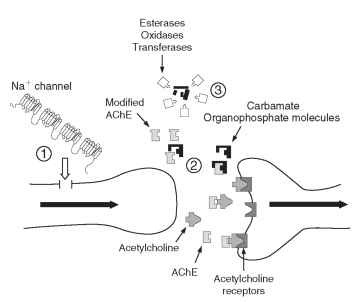
FIGURE 2 Schematic diagram of a nerve synapse showing examples of insecticide resistance mechanisms: (1) changes in the structure of the sodium channel confer kdr or super-kdr target-site resistance to pyrethroids; (2) modified AChE is no longer bound by organophosphates and remains available to break down acetylcholine molecules after neurotransmission across the synapse; (3) detoxifying enzymes degrade or sequester insecticides before they reach their targets in the nervous system.
flour beetle, Tribolium castaneum), other examples suggest that some mechanisms have arisen only once. Of the many Organophosphate resistance alleles found in the mosquito Culex pipiens, the allele Ester2 has a unique global distribution. It has been shown that this is the result of extensive migration (natural and human-assisted) by the insect and not due to independent evolution at multiple geographical locations. Its spread has probably been aided by human activity, as mosquitoes are common stowaways in ships and planes. It is interesting to consider why Ester2 is more widely distributed than other allele variants. It might be the result of sheer chance, or of it being the first mechanism to evolve (i.e., it has had more time to spread and establish), or of it being the fittest variant (i.e., it might confer greater resistance in the presence of OPs or be the least costly in the absence of exposure). In fact, the situation is complex. There are fitness costs associated with Ester2 that preclude its existence at high frequencies in nontreated areas; but in areas of moderate OP pressure, it outcompetes and replaces other allelic variants. Its global distribution, therefore, results from human-aided dispersal whose patterns are influenced by a dynamic mixture of insecticide selection and intrinsic fitness cost. Studies on aphids and whiteflies also suggest single origins for resistance mutations that are now geographically very widespread, presumably as a consequence of their dispersal via the global trade in plant material contaminated with pest organisms.
FACTORS AFFECTING THE EVOLUTION OF RESISTANCE
As an evolutionary trait, insecticide resistance is unusual in that we can identify the main selection pressure with ease, but the rate at which resistance develops is governed by numerous biotic and abiotic factors. These include the genetics and ecology of the pests and their resistance mechanisms, and the operational factors that relate to the chemical itself and to its application. To manage resistance effectively, an assessment of genetic, ecological, and operational risk is required. Although this can be done empirically on a species-by-species basis, one of the great challenges of the future is to understand why some species seem to have a greater tendency to become resistant than others.
Genetic Influences
To predict how quickly resistance will become established, it is necessary to understand how resistant alleles affect the survival of phenotypes in the field. For example, the dominance of resistance genes exerts a major influence on selection rates. In laboratory bio-assays evaluating the relative survival of susceptible homozygotes (SS), heterozygotes (RS), and resistance homozygotes (RR) over several insecticide concentrations, RS individuals usually respond in an intermediate manner. In the field, however, dominance is dependent on the concentration of insecticide applied and its uniformity over space and time. Even when the initial concentration is sufficient to kill RS individuals (rendering resistance effectively recessive), upon weathering or decay of residues, this genotype may later show increased survival, with resistance becoming functionally dominant in expression. When resistance genes are still rare, hence mainly present in heterozygous condition, this sequence can have a profound effect in accelerating the selection of resistance genes to economically damaging frequencies.
The diverse mating systems of insects also influence the rate at which resistance evolves. Although most research has focused on outcrossing diploid species (typified by members of the Lepidoptera, Coleoptera, and Diptera), systems based on haplodiploidy and parthenogenesis also occur among key agricultural pests. In haplodiploid systems, males are usually produced uniparentally from unfertilized, haploid eggs, and females are produced biparentally from fertilized, diploid eggs. The primary consequence of this arrangement (exemplified by whiteflies, spider mites, and phytophagous thrips) is that resistance genes are exposed to selection from the outset in the hemizygous (haploid) males, irrespective of intrinsic dominance or recessiveness. Whether a resistance gene is dominant, semidomi-nant, or recessive, resistance can develop at a similar rate.
Most species of aphid undergo periods of parthenogenesis (in which eggs develop and give rise to live offspring in the absence of a paternal genetic contribution) promoting the selection of clones with the highest levels of resistance and/or the most damaging combination of resistance mechanisms. In fully anholocyclic (asexual) populations, such as those of M. persicae in northern Europe, the influence of parthenogenesis has led to strong and persistent associations between resistance mechanisms within clonal lineages.
Ecological Influences
Fecundity and generation times have a huge bearing on the evolution of resistance in a population. The greater the number of individuals, and the faster they reproduce and attain maturity, the higher the likelihood that a favorable mutation will occur and be maintained in the population. Faster growth and higher population numbers will affect the size of a pest population and therefore the need for and frequency of insecticide treatment.
The dispersal capabilities of pests can also act as primary determinants of resistance development. Movement of pests between untreated and treated parts of their range may delay the evolution of resistance because of the diluting effect of susceptible immigrants. Conversely, large-scale movement can also accelerate the spread of resistance by transferring resistance alleles between localities. An example of how dispersal is key to the development of resistance management strategies relates to the control of two major cotton bollworm species (Lepidoptera: Noctuidae) in Australia. One (H. armigera) is a cosmopolitan pest whilst the other (H. punctigera) is endemic to Australia with a wider range of native, non-crop hosts. Historically, H. armigera has proved an almost intractable problem, rapidly developing resistance to many insecticide classes. Conversly, Helicoverpa punctigera, an equally important cotton pest, has remained susceptible to all insecticide classes, probably because its greater host range (and long-range dispersal between these hosts) results in the maintenance of a large pool of unselected, susceptible individuals, which dilute any resistant mutations that arise.
Operational Influences
Operational factors are at human discretion and can be manipulated to influence selection rates. Factors exerting a major influence in this respect include the rate, method, and frequency of applications, their biological persistence, and whether insecticides are used singly or as mixtures of active ingredients.
Equating operational factors with selection is often difficult, because without detailed knowledge of the mechanisms present it is impossible to test many of the assumptions on which genetic models of resistance are based. If resistance alleles are present, the only entirely nonselecting insecticide doses will be ones sufficiently high to overpower all individuals, regardless of their genetic composition, or ones so low that they kill no insects at all. The latter is obviously a trivial option. Prospects of achieving the former depend critically on the potency and dominance of resistance genes present. A pragmatic solution to this dilemma is to set application doses as far above the tolerance range of homozygous, susceptible individuals as economic and environmental constraints permit, in the hope that any heterozygotes that do arise will be effectively controlled. However, this approach will obviously be ineffective if resistance turns out to be more common than suspected (resulting in the presence of homozygous resistant individuals) or if resistance alleles exhibit an unexpectedly high degree of dominance (and heterozygotes are therefore phenotypically resistant). Unless a high proportion of insects escape exposure altogether, the consequence could then be very rapid and effective selection for homozygous resistant populations.
In practice, concerns about optimizing dose rates to avoid resistance are secondary to those related to the application process itself. Delivery systems and/or habitats promoting uneven or inadequate coverage will generally be more prone to select for resistance, because, under these circumstances, pests are likely to encounter suboptimal doses of toxins that will permit survival of heterozygous individuals.
The timing of insecticide applications relative to the life cycle of a pest can also be an important determinant of resistance. Targeting of insecticides against newly hatched larvae, as is generally advocated for bollworm control, not only increases the likelihood of contacting larvae at the most exposed stage in their development but also offers the greatest prospect of retarding resistance by overpowering its expression (the sensitivity of larvae to insecticides declines with increasing size). It may also have the effect of reducing genetic variation and therefore the potential number of resistant mutations. Indeed, it is also possible to impose genetic “bottlenecks” by applying pesticides when populations are already low (e.g., when they are overwintering). Although such a tactic might be beneficial where populations are fully susceptible, if resistant mutations are already present, it might act to increase their frequency.
In theory, the application of two or more unrelated chemicals as insecticide mixtures offers substantial benefits for delaying the selection of resistance. The underlying principle is one of “redundant killing,” whereby any individuals already resistant to one insecticide are killed by simultaneous exposure to another, and vice versa. However, achieving this objective requires that each type of resistance be rare and that both ingredients persist throughout the effective life of an application. Otherwise, one compound will exert greater selection pressure than the other, and the advantage of applying a mixture will be lost.
Fitness of Resistant Individuals
In the absence of insecticidal selection pressure, resistance genes can impose fitness costs on their carriers. Sometimes these costs are quite subtle and difficult to determine. In M. persicae, a reduced response to alarm pheromone (produced as a warning signal by aphids) was associated with both the amplification of a gene that codes for OP resistance and the kdr target-site mutation. In this species, gene amplification was also associated with a decreased propensity to move from senescing leaves to fresh leaves at low temperature and hence reduced overwintering survival. Housefly genotypes possessing the identical kdr mutation were shown to exhibit behavioral differences in comparison with susceptible insects. Resistant individuals showed no positional preference along a temperature gradient while susceptible genotypes exhibited a strong preference for warmer temperatures. Costs such as these can contribute to a decline in the frequency of resistant insects when insecticide pressure is relaxed. However, reduced fitness is by no means an inevitable side effect of acquiring resistance. There are examples of mutations at even highly conserved sites in essential proteins that seemingly exert no effect on the fecundity, development, or survival of their carriers.
COMBATING INSECTICIDE RESISTANCE
Insecticide resistance management (IRM) aims to intervene in the evolutionary process and either overcome resistance or prevent its appearance in the first place. There are several practical, economic, and political constraints on the choice of possible IRM tactics and the precision with which they can be applied:
• The properties of any resistance genes present are often unknown, and knowledge of pest ecology may still be rudimentary.
• It is often necessary to contend with a whole pest complex rather then just a single pest species.
• There may be a very limited number of insecticides available for use in management strategies.
• For highly mobile pests, at least, countermeasures may need to be standardized and synchronized over large areas, sometimes whole countries.
• Resistance is a dynamic phenomenon; that is, any mechanisms already known to exist may change over time.
• To promote compliance with management strategies, the tactics adopted should be as unambiguous, rational, and simple as possible.
A strategy first implemented on Australian cotton in 1983 against H. armigera illustrates many features of large-scale attempts at resistance management. Introduced in response to unexpected, but still localized, outbreaks of pyrethroid resistance in H. armigera, the strategy was based primarily on the concept of insecticide rotation. The threat of pyrethroid resistance was countered by restricting these chemicals to a maximum of three sprays within a prescribed
time period coincident with peak bollworm damage. To diversify the selection pressures being applied, farmers were required to use alternative insecticide classes at other stages of the cropping season. Additional recommendations, including the targeting of insecticides against newly hatched larvae (the most vulnerable life stage), the plowing in of cotton stubble to destroy resistant pupae overwintering in the soil, and the use of trap crops to concentrate pests in particular areas where they could then be controlled by other means (i.e., destructive cultivation of the crop), were major additional components of the program. The result was to delay but not prevent the evolution of resistance to the pyrethroids. These days, pyrethroids remain among the insecticides listed for rotation with organophosphates, carbamates, and a range of newer products that have been introduced since the strategy was first devised. Management of resistance to conventional insecticides remains vital to cotton production alongside tactics for combating pest resistance to transgenic crops expressing Bt toxins (see below).
A major innovation in crop protection has been the release of crop plants genetically engineered to express genes for insecticidal Bt toxins derived from the microbe B. thuringiensis. Existing toxin genes in Bt cotton and corn are active specifically against certain key lepi-dopteran pests (especially bollworms and corn borers), and another has been engineered into potatoes to provide protection against the Colorado potato beetle (but due to poor sales, all genetically engineered potato varieties have now been discontinued). The production of Bt non-food crops continues to accelerate. In 2006, 19.0 million hectares were planted with Bt crops and 13.1 million hectares with the stacked traits of Bt and herbicide tolerance. Aside from their commercial prospects, insect-tolerant transgenic crops offer numerous potential benefits to agriculture. By affording constitutive expression of toxins in plant tissues throughout a growing season, the incorporation of Bt genes into crops should reduce dramatically the use of conventional broad-spectrum insecticides against insect pests and therefore environmental contamination. It removes the dependence of pest control on extrinsic factors such as climate and on the efficiency of traditional application methods. However, this high and persistent level of expression also introduces a considerable risk of pests adapting rapidly to resist genetically engineered toxins. To date, there are no substantiated reports of resistance selected directly by exposure to commercial transgenic crops, but resistance to conventional Bt sprays (selected in either the laboratory or the field) has been reported in more than a dozen insect species. Research into the causes and inheritance of such resistance provides valuable insights into the threats facing Bt plants and the efficacy of possible counter-measures. Tactics proposed for sustaining the effectiveness of Bt are more limited in scope than for conventional insecticides because of the long persistence and constitutive expression of engineered toxins and because of the limited diversity of transgenes currently available. Indeed the only prudent and readily implementable tactic currently employable is to ensure that substantial numbers of pests survive in nontransgenic refugia, a stategy dependent on some key assumptions: (1) that resistant mutations are recessive or at least only partially dominant, so that their heterozygous forms can be controlled by the toxins expressed; (2) that refugia will produce enough susceptible insects to ensure that insects carrying resistant alleles do not meet and mate; and (3) that resistant alleles will carry a fitness cost, rendering insects less fit when the selection pressure is removed (e.g., outside the growing season when the insect is dependent on other crops).
As an example of a transgenic crop, the majority of cotton grown in Australia is now engineered to express two Bt toxins (Cry1Ac and Cry2Ab). These toxins are lethal to all field populations of cotton boll-worm (H. armigera). In order to preserve this susceptibility, each Bt cotton farmer is legally required to grow a refuge crop capable of producing sufficient Bt susceptible Helicoverpa moths to dominate any mating interactions with survivors emerging from Bt crops. It is hoped that stacking (or pyramiding) these two genes in the same cultivar, in combination with the use of refugia, will result in a far more durable resistance management strategy than has been afforded by the presence of single Bt toxins. It is clearly essential that plants expressing insecticidal transgenes (essentially analogous to conventionally-bred pest-resistant cultivars) be exploited as components of multitactic strategies rather than as a panacea for resistance problems with conventional insecticides.
RESISTANCE IN NONPEST SPECIES
In contrast to the detrimental impact of resistance in pests, the ability of insect predators and parasitoids to develop pesticide resistance could be of enormous benefit to pest management strategies incorporating insecticides. Although pyrethroid and organophosphate resistance has been documented in predatory mites (e.g., Typhlodromus pyri in orchards and Amblyseius womersleyi in tea fields) and hymenopterous parasitoids (e.g., Aphytis holoxanthus in orchards and Anisopteromalus calandrae in grain stores), reports of insecticide-resistant beneficial species from the field are far rarer than they are for pest species. Reporting bias aside, the most likely reasons for this are the difficulty in host location when both natural enemy and host are under selection pressure and, in comparison with herbivorous species, the possibility that the enzyme systems of predators and parasites are less well adapted to detoxify xenobiotics.
In general, when resistance does occur in nonpest species, its mechanisms are similar to those exhibited by pest species. Organophosphate resistance in strains of A. calandrae has been linked to the presence of carboxylesterase-like enzymes similar to those conferring organophosphate resistance in the aphid M. persi-cae. The expression level of the carboxylesterase-like enzyme in this wasp is approximately 30-fold higher in the resistant strain relative to that in the susceptible strain, and the mechanism seems to have its basis in a single-nucleotide replacement. Organophosphate resistance in strains of the warehouse pirate bug (Xylocoris flavipes) has also been linked to the presence of a carboxylesterase. Resistance to this chemical group in the lacewing, Chrysopa scelestes, has been attributed to increased AChE activity.
CONCLUDING REMARKS
Research on the topic of insecticide resistance has provided invaluable insights into the origin and nature of adaptations, and these are proving to have broad significance for understanding genetic responses to man-made change in the environment. In many respects, the continuing battle against resistance is as good an example of coevolution as any and is a clear illustration of how such processes influence biological diversity.
It is important to note that the pest management problems posed by the evolution of resistance are not unique to control strategies that use conventional insecticides. Codling moths (Cydia pomonella) were recently reported to have evolved up to 100,000-fold resistance to a baculovirus applied as a biological control agent that was widely considered to be much less vulnerable to resistance development. The utilization of host plant resistance is another case in point. Resistance to insects in crop plants is selected by screening for genes that provide resistance in the laboratory or in field plots, and then inserting those genes into crop strains with other desirable characteristics (through crossing or genetic engineering). At least eight major genes for resistance to the Hessian fly (Mayetiola destructor) have been successively bred into wheat over the past two decades. In each instance, the introductions of new insect-resistant mutations in the plant were rendered useless by the evolution of corresponding protective adaptations in the fly. Another example of such coevolution comes from the use of semiochemical tools for pest control. In many parts of Asia, a synthetic pheromone is used to disrupt mating in a tea tortrix moth (Adoxophyes honmai), the larvae of which can cause severe damage in tea plantations. Researchers in Japan recently reported the evolution of a new biotype of this species that exhibits reduced sensitivity to mating disruption. In this case, resistance may have evolved quickly because the original formulation used did not match the precise blend used by the female. Use of the complete pheromone blend restored efficacy. Such events make it clear that regardless of whether the major strategies for pest management continue to use conventional chemicals, the “arms race” between insect evolution and human ingenuity will continue to present major challenges.
