Mayflies (order Ephemeroptera) date from Carboniferous and Permian times and represent the oldest order of the existing winged insects. They are unique among the insects in having two winged adult stages, the subimago and imago (Fig. 1 ). Adult mayflies do not feed; instead, they rely on reserves built up during their nymphal life. As adults they generally live from 1 to 2 h to a few days, and mayflies spend most of their life in the aquatic environment, either as eggs or nymphs. The nymphal life span in mayflies varies from 3 to 4 weeks to more than 2 years. The length of egg development varies from ovoviviparity (i.e., the release of live offspring) to a period of up to 10 to 11 months in some arctic/alpine species.
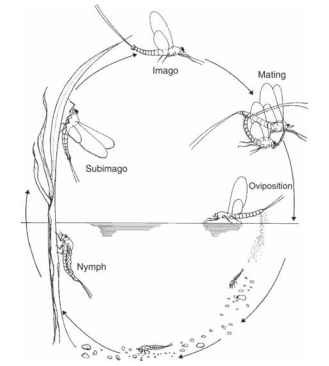
FIGURE 1 Mayfly life cycle showing the alternation between the aquatic and terrestrial environments. Mayflies are unique in having two winged stages, the subimago and imago. The adult life is very short and most of the time is spent in the aquatic environment.
Because of their winged adult stage and a propensity for drift (i.e., downstream movements) as nymphs, mayflies are often among the first macroinvertebrates to colonize virgin habitats. However, over longer distances their dispersal capacity is limited, owing to their fragility and short adult life. Mayflies are found in almost all types of freshwater habitat throughout the world, although in the Arctic and in mountain areas above the tree line there are few species. Mayfly faunas on oceanic islands and isolated mountain areas have few species, and they are usually restricted to the Baetidae and/or Caenidae. Their greatest diversity is in lotic habitats in temperate and tropical regions, where they are an important link in the food chain, from primary production by algae and plants to secondary consumers such as fish. Mayflies are used extensively as indicators of pollution and environmental change.
ORIGINS AND EVOLUTION
Ephemeroptera are among the oldest known winged insects still extant. Carboniferous fossils have been ascribed to mayfly precursors or even mayflies. Permian data confirm that the order was already present at the end of the Paleozoic. Ephemeroptera reached their highest diversity during the Mesozoic, mainly in the Jurassic and Cretaceous. All these species belong to extinct families. The Tertiary fauna, as documented by, for instance Baltic amber, is undeniably modern, with both the extinct and living genera of modern families.
The relationship of Ephemeroptera with other modern winged insects is still a subject of debate. Together with the Odonata, mayflies were traditionally placed in the Paleoptera, which was considered the sister group of all other extant primarily winged orders. More recently, it was suggested that Ephemeroptera per se are the sister group of Odonata + Neoptera. This idea is based on a number of features unique to mayflies, such as the presence of a subimaginal stage, the nonfunctionality of the adult mouthparts, and the presence of only one axillary plate in the wing articulation. This hypothesis is also supported by anatomical data: female mayflies exhibit telotrophic meroistic ovaries instead of panoistic ones as found in Odonata.
CLASSIFICATION AND PHYLOGENY
The Ephemeroptera are numerically a small order of insects, with about 3100 described species within more than 400 genera and 40 families (Table I). About 350 species occur in Europe, and 650 in North America. During the last 20 years, partly as a result of the discovery of new taxa, especially in tropical areas, where the mayfly fauna is still poorly known, 10 new families, 90 genera, and more than 500 species have been added. The expansion of the order is also the result of several phylogenetic analyses that led to a narrower concept of supraspecific taxa. As a consequence of these important analyses, there is no real consensus about the higher classification of Ephemeroptera (superfamilies, suborders, or infraorders). Based on the structure of the nymphal wing pads, mayflies were traditionally divided into two suborders, Pannota (with fused wing pads) and Schistonota (with free wing pads). That the latter suborder is para-phyletic is now well documented, but there is no agreement about the composition and even the names of these higher taxa.
ADULTS
The adult mayfly has two main functions, mating and oviposition, which produce a general uniformity in structure. The prominent
TABLE IOverview of the Mayfly Families and the Number of Genera and Species as of December 31, 2007 |
|||
| Family | Genera | Species | Biogeography |
| Acanthametropodidae | 2 | 3 | Asia and North America |
| Ameletidae | 2 | 56 | Asia, Europe, and North America |
| Ameletopsidae | 4 | 6 | Australia, New Zealand, and South America |
| Ametropodidae | 1 | 3 | Asia, Europe, and North America |
| Baetidae | 96 | 860 | Worldwide |
| Baetiscidae | 1 | 12 | North America |
| Behningiidae | 3 | 7 | Asia, Europe, and North America |
| Caenidae | 18 | 211 | Worldwide |
| Coloburiscidae | 3 | 6 | Australia, New Zealand, and South America |
| Coryphoridae | 1 | 1 | South America |
| Dipteromimidae | 1 | 2 | Japan |
| Ephemerellidae | 18 | 160 | Worldwide except Australia and New Zealand |
| Ephemeridaea | 7 | 91 | Worldwide except Australia and New Zealand |
| Ephemerythidae | 2 | 5 | Continental Africa |
| Euthyplociidae | 5 | 19 | Asia, Madagascar, and South America |
| Heptageniidaeb | 34 | 529 | Africa, Asia, Europe, and North America |
| Ichthybotidae | 1 | 2 | New Zealand |
| Isonychiidae | 1 | 30 | Asia, Europe, North and South America |
| Leptohyphidae | 13 | 157 | North and South America |
| Leptophlebiidae | 134 | 623 | Worldwide |
| Melanemerellidae | 1 | 1 | South America |
| Metretopodidae | 3 | 13 | Asia, Europe, and North America |
| Neoephemeridae | 3 | 11 | Asia, Europe, and North America |
| Nesameletidae | 3 | 7 | Australia, New Zealand, and South America |
| Oligoneuriidaec | 13 | 54 | Worldwide except Australia and New Zealand |
| Oniscigastridae | 3 | 8 | Australia, New Zealand, and South America |
| Palingeniidae | 7 | 32 | Asia, Europe, and Madagascar |
| Polymitarcyidaed | 7 | 84 | Worldwide except Australia and New Zealand |
| Potamanthidae | 3 | 23 | Africa, Asia, Europe, and North America |
| Prosopistomatidae | 1 | 19 | Africa, Asia, Australia, Europe, and Madagascar |
| Rallidentidae | 1 | 1 | New Zealand |
| Siphlaenigmatidae | 1 | 1 | New Zealand |
| Siphlonuridae | 4 | 49 | Asia, Europe, and North America |
| Teloganellidae | 1 | 1 | Southeast Asia |
| Teloganodidae | 7 | 13 | Africa, Asia, and Madagascar |
| Tricorythidaee | 6 | 34 | Africa, Asia, and Madagascar |
| Vietnamellidaef | 2 | 7 | Asia and Australia |
| Total | 414 | 3142 | |
turbinate eyes of males, especially well-developed in the Baetidae and some Leptophlebiidae, provide both high acuity and good sensitivity. This enables them to detect and capture single females in a swarm at low light intensities.
The forelegs of most mayflies also show sexual differences; those of the male are unusually long for grasping and holding the female during mating. In the Polymitarcyidae, the middle and hind legs of the male and all the legs of the female are reduced, and in Dolania (Behningiidae) all the legs of both sexes are reduced. In Dolania and several members of the Polymitarcyidae and Palingeniidae, the females remain in the subimaginal stage. The reason for two winged stages has provoked much discussion. It has been suggested that this primitive trait is maintained because there has not been the selective pressure on the short-lived stages to produce just a single molt. Another explanation is that two molts are necessary to complete the elongation of the caudal filaments and forelegs of the adults. Most mayflies have two pairs of wings, but in the Caenidae, Tricorythidae, Baetidae, and some Leptophlebiidae, the hind wings are reduced or even absent.
Fecundity
Spermatogenesis and oogenesis are generally completed in the final nymphal instar, and the eggs and sperm are physiologically mature in the subimago. Most species produce 500-3000 eggs, but values range from less than 100 in Dolania to 12,000 in Palingenia, and the fecundity values recorded for the females of the larger species of mayfly are higher than in most other insect groups except the social Hymenoptera. In species with a long emergence period or with a bivoltine life cycle (having two summer emergence periods), early emerging females are larger and therefore more fecund than those emerging later.
MATING AND SWARMING
Swarming in adults is a male activity, apart from the Caenidae and Tricorythidae, where both males and females may participate. The females fly into these swarms, and mating occurs almost immediately and usually in flight. Swarming may take place over the water itself, over the shore area, or even away from the water. Most swarms are positioned according to terrain markers such as areas of vegetation, the shoreline, and trees. The time of swarming varies considerably, although dusk is the most common time of day in temperate regions. Parthenogenesis has been reported in about 50 mayfly species, although it is not obligatory as a rule.
Oviposition
The majority of mayflies, including most Ephemeridae, Heptageniidae, and Leptophlebiidae, oviposit by descending to the water and releasing a few eggs at a time by dipping their abdomen into the water. Species of Ephemerella, Siphlonurus, and Centroptilum, however, release all their eggs in a single batch that separates immediately on contact with water. In Habroleptoides and some Heptageniidae the female rests on a stone above the water, and dips her abdomen into the water to lay the eggs. This is taken a stage further in several species of Baetis in which the female actually goes underwater and lays her eggs on suitable substrate, often under stones.
EGGS
Mayfly eggs have a variety of attachment structures that enable them to adhere to submerged objects or to the substrate. Differences in egg morphology have enabled the construction of identification keys, purely on the basis of eggs. This has provided a useful complement,
not only to studies of phylogeny, but also to taxonomy, since identification of female adults by means of external characters is often difficult.
Development
Most nymphs hatch at temperatures in the range of 3-21°C. However, in the North American Hexagenia rigida, the nymphs hatch successfully between 12 and 32°C and even at 36°C if incubation is started at lower temperatures. In Tricorythodes minutus, nymphs hatch between 7.5 and 23°C, but mortality is least at 23°C. Hatching success is variable, ranging from over 90% in several Baetis and Hexagenia species to less than 50% in the Heptageniidae studied. Excluding the few ovoviviparous species, the total length of the egg development period varies from a week in H. rigida to almost a year in Parameletus columbiae. Temperature is the major factor determining the length of the period of egg development in mayflies. There is no indication that photoperiod influences egg development time. Ovoviviparity is rare in the mayflies and is restricted to the Baetidae. In North America, a number of species in the genus Callibaetis are ovoviviparous.
NYMPHS
In contrast to the adults, mayfly nymphs show considerable diversity in habit and appearance. Differences do not always follow taxonomic lines, and convergent and parallel evolution seems to be common ( Fig. 2 ).
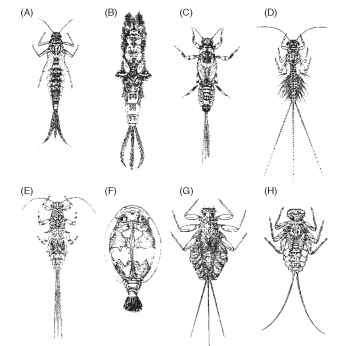
FIGURE 2 Mayfly nymphs: (A) Baetis subalpinus (family Baetidae), (B) Ephemera danica (family Ephemeridae), (C) Ephemerella mucro-nata (family Ephemerellidae), (D) Leptophlebia vespertina (family Leptophlebiidae), (E) Caenis robusta (family Caenidae), (F) Prosopistoma boreus (family Prospistomatidae), (G) Lepeorus thi-erryi (family Leptophlebiidae), and (H) Epeorus alpicola (family Heptageniidae). Illustrations show some of the large range in morphology, often related to habitat and food habits and not necessarily to family relationships. For example, L. thierryi and E. alpicola are morphologically similar and adapted to fast-running waters but belong to different families.
Growth and Development
Mayflies have a large number of postembryonic molts. Estimates of the number of instars vary between 10 and 50; most are in the range 15-25. The number of instars for a particular species does not seem to be constant, but probably varies within certain limits. Environmental conditions, such as food quality and temperature, may affect instar number. Because of its simplicity, by far the most common measure of development and growth in mayflies has been body length, although head width and other body dimensions also have been used. However, growth of the various body parts is not always isometric. Many authors have also used body weight, and the length-weight relationship is usually well expressed by a power function.
Nymphal growth rates are influenced by several environmental factors, although the major growth regulator is mean temperature, the scale of diurnal fluctuations, or total degrees-days. Other factors, such as food and current velocity, may exert a modifying influence on growth rates. No true diapausing nymphal stage has been reported in the Ephemeroptera, although growth rates often are very low during the winter.
Respiration
The gills of mayflies are very diverse in form, ranging from a single plate in Ameletus to fibrillar tufts in Hexagenia. Respiratory tufts are sometimes developed on other parts of the body besides the abdomen, such as those at the base of the coxa in Isonychia and Dactylobaetis. In several families, the second abdominal gill has developed into an operculate (lidlike) gill cover for the remaining gills, and in certain Heptageniidae the gills are markedly expanded so that they together form an adhesion disc. In many of the Siphlonuridae, the gills are used as swimming paddles, which has been put forward as their original function. In respiring, the gills may function either as respiratory organs or as ventilatory organs for other respiratory exchange surfaces.
High rates of oxygen consumption are often reported in association with emergence and gonad maturation. High water temperatures at that time may mean that low oxygen concentrations can be critical. Many burrowing Ephemeridae and pond-dwelling Baetidae are able to survive moderately low oxygen concentrations, especially for short periods. However, so far only one species, the European Cloeon dipterum, has been shown to survive long-term anoxia.
Population Movements
During the final stages of nymphal life there is a movement to and a concentration in the shallower areas of lakes and rivers. In running waters, springtime mass movements of mayfly nymphs along the banks of the main river and into slower flowing tributary streams or into areas flooded by spring snowmelt have been observed. In running water, mayfly nymphs may move down into the substratum in response to spates or as part of a daily rhythm. Generally, however, mayflies do not extend far down into the substratum (i.e., the hyporheic zone).
Mayflies, especially Baetidae, are a major component of invertebrate drift in running waters. Their drift shows a strong diel periodicity, with a peak during the hours of darkness. Drift rates are not constant for a particular species, and the larger size classes are usually more in evidence. Other factors that have been shown to influence mayfly drift include changes in current velocity and discharge, increased sediment loading, temperature changes, oxygen conditions, density, food availability, and predators.
EMERGENCE
Emergence, the transition from the aquatic nymph to the terrestrial subimago, is a critical period for mayflies. Their movement up to the water surface makes them especially vulnerable to aquatic and aerial predators. Shedding of the nymphal skin usually occurs at the water surface on some object, such as a stone or macrophyte stem, or in midwater. The latter location is more typical of the burrowing species that inhabit deeper waters and of a number of river species. Genera such as Siphlonurus, Isonychia, and Baetisca crawl completely out of the water before they molt.
Diel Patterns
In temperate regions, the crepuscular emergence of mayflies is well known. However, dusk is not the only time of day that mayflies emerge, although most species exhibit clear diel patterns of emergence that are, with few exceptions, characteristic for a given species, genus, or even a whole family. For example, the emergence of the short-lived Caenidae invariably takes place either at dawn or dusk and seems to be controlled by light intensity. Several baetid and leptophlebiid genera emerge around midday. In temperate areas, the higher daytime air temperatures are less restrictive for flight activity, although the adults are probably more susceptible to predation.
In the tropics and warm temperate regions, night air temperatures are less restrictive, and to escape from daytime predators it seems that most longer-lived forms emerge during the first 2h of darkness. The shorter-lived genera, such as Caenis, are subject to fewer restraints on their emergence, and there are few constant differences between tropical and temperate species.
The daily emergence of males and females is usually synchronous, especially in the short-lived forms, although there may be an excess of males at the start of the day’s emergence. In species in which the females oviposit as subimagos, the males, which molt to imago, emerge well before the females.
Seasonal Patterns
Mayflies have distinct and finite emergence periods, especially in temperate and arctic areas. In the tropics, emergence is often non-seasonal, although some species have clear emergence patterns. The lunar rhythm of emergence of the African species, Povilla adusta, from a number of lakes is well known. The burrowing mayflies of the Ephemeridae, Polymitarcyidae, and Oligoneuriidae are noted for their sporadic mass emergence. The mass emergence of Hexagenia from the Mississippi River has been well documented. There are latitudinal and altitudinal gradients in the timing of emergence. For example, in both North American and European Leptophlebia, emergence occurs progressively later as one moves northward. In a similar way, the onset of emergence is delayed with increasing altitude. In habitats with several mayfly species, peak emergence of the major species may be separated in time, especially in congeneric species.
It has been suggested that emergence falls into two main categories: synchronized and dispersed, and represents two approaches for reducing adult mortality. Synchronous emergence attempts to saturate a potential predator, and dispersed emergence seeks to lower the possibility of predator-prey encounters. However, emergence pattern can vary with abundance and locality, and from year to year within the same species.
Water temperature thresholds, often in conjunction with rising temperatures, are important for both seasonal and daily emergence of many mayflies. Photoperiod has also been suggested as a potential
factor regulating seasonal emergence in mayflies; few concrete data are available, however, and successful emergence occurred when nymphs were reared in complete darkness. Other abiotic factors may also affect daily emergence totals.
LIFE CYCLES
There is an extensive literature on mayfly life cycles, although mostly from temperate areas in Europe and North America. However, care should be taken in the interpretation of mayfly life cycles, especially when only field observations are available. Particular care is necessary in interpreting the length of time for egg development from field data.
Several authors have classified mayfly life cycles; most have used a combination of voltinism, duration of egg development, and nym-phal growth rates as criteria. Multivoltine species usually have two or three generations in temperate regions, often a slowly growing winter generation and one or two rapidly growing summer generations. Limited data from the tropics, where many species are nonseasonal, indicate that some species go through about four and possibly up to six generations during the course of a year.
In temperate areas, the univoltine life cycle is the most widespread type. Several authors have distinguished two main types of univoltine cycle: when overwintering occurs during the nymphal stage after a relatively short egg developmental period, and when hatching occurs in the spring after a long period of egg development. Semivoltinism, with generation times up to 3 years, is relatively uncommon in mayflies.
Mayfly life cycles show a distinct trend from the tropics to the Arctic. In the tropics, nonseasonal multivoltine cycles predominate, with seasonality becoming more distinct in mountainous and continental areas. As one approaches the Arctic, univoltine cycles dominate.
Many mayflies exhibit flexibility in life cycle, whereas some mayflies (e.g., the widespread species Leptophlebia cupida) have a univoltine winter cycle over a wide range of latitudes and climates. However, a number of common and widespread species display a considerable degree of life cycle flexibility throughout their distributional range. This is perhaps best exemplified by many Baetidae, which may switch from multivoltine to univoltine depending on climate. The North American Hexagenia show a similar flexibility.
ABIOTIC AND BIOTIC RELATIONSHIPS
The majority of mayfly nymphs are herbivores, feeding on detritus and periphyton (algal communities on stones and plants). This explains their relative uniformity in mouthparts. The modifications that are present are a result of different food-gathering mechanisms rather than differences in diet. The herbivorous mayflies fall into two main categories, collectors and scrapers. Among the collectors, several genera are filter feeders, with setae on the mouthparts or forelegs acting as filters. Oligoneuriidae, Leptophlebiidae, Siphlonuridae, and the Heptageniidae have several genera that are probably filter feeders. By using their gills to produce a current of water through their burrows, several of the Ephemeridae and Polymitarcyidae may, at least for part of their food supply, be regarded as filter feeders. To supplement their diet, Povilla nymphs, especially the larger ones, leave their burrows at night and graze on periphyton. Most mayflies, however, are fine-particle detritivores. These include many Siphlonuridae, Baetidae, Leptophlebiidae, Metretopodidae, Ephemerellidae,
Caenidae, and Baetiscidae, as well as some Heptageniidae. Members of the other major feeding group within the mayflies, scrapers, feed on the periphyton present on mineral and organic surfaces. These include representatives of several mayfly families, notably the Baetidae, Heptageniidae, Leptophlebiidae, and Caenidae. Shredders are probably also represented among mayflies.
True omnivory is of limited occurrence in the mayflies and is restricted to some species in genera such as Isonychia, Siphlonurus, Stenonema, and Ephemera. The predatory habit is also relatively uncommon in the mayflies. In North America, Dolania, Analetris, and the heptageniids, Pseudiron, Spinadis, and Anepeorus, feed largely on chironomids. The baetid genera Centroptiloides and Raptobaetopus have carnivorous nymphs. Within the Prosopistomatidae there are also carnivorous species. Several species, such as Siphlonurus occidentalis and Stenonema fuscum, may change from a predominantly detrital diet in the early instars to one containing a significant proportion or even a dominance of animal material in the mature nymphs.
The time for food to pass through the gut is often short, and in Baetis, Cloeon, and Tricorythodes, it has been shown to be only about 30 min. Hexagenia nymphs feed continuously during the day and night, and at most temperatures they ingest over 100% of their dry body weight per day. In contrast, values for the surface-dwelling collector Stenonema are much lower and vary between 2 and 22% of dry body weight per day. The carnivorous Dolania, feeding more intermittently but on a higher energy diet, has consumption indices similar to those of Stenonema. Studies have shown little or no cel-lulase activity in mayflies, whereas the proteolytic activity of trypsin-and pepsinlike enzymes is very high.
Predation
Mayfly nymphs are eaten by a wide range of aquatic invertebrate predators, including stoneflies, caddisflies, alderflies, dragon-flies, water beetles, leeches, triclads, and crayfish. Mayflies are also important food organisms for fish. Birds and winged insects, such as Odonata, also prey on mayfly adults. Birds may take both the aquatic nymphs and the aerial adults. Several other animal groups, including spiders, amphibians, marsupials, and insectivorous mammals such as bats and shrews, have been reported to take mayflies. Many parasites also utilize these food chain links.
Symbiosis, Phoresy, and Parasitism
There is a wide range of organisms that live on or in mayflies. They include the normal spectrum of protozoan, nematode, and trematode parasites, and phoretic and commensal relationships with other organisms occur, as well. Chironomids in the genus Symbiocladius are ectoparasites and may cause sterility, although ectoparasites in the genus Epoicocladius do not seem to be detrimental to their host. In fact the cleaning effect, especially of the gills, may facilitate oxygen uptake in the mayfly. Mayflies can also be commensal, and two baetid genera, Symbiocloeon from Thailand and Mutelocloeon from West Africa, live between the gills of freshwater mussels.
DISTRIBUTION AND ABUNDANCE
Because of their fragility and short adult life, mayflies are generally rather limited in their dispersal powers. Together with their ancient origin and the strict association of larvae with fresh-waters habitats, Ephemeroptera represent an interesting group for biogeographical analyses. The Siphlonuridae and allied families, typically cool-adapted mayflies, are mainly distributed in the temperate Northern Hemisphere, except for the Oniscigastridae, Nesameletidae, Rallidentidae, and Ameletopsidae, which are confined to New Zealand, Australia, and southern South America. We can hypothesize that this lineage was already present on the Pangaea, and radiated later on in Laurasia (Northern Hemisphere continent). Gondwanian representatives (Southern Hemisphere continent) expanded over the transant-arctic land bridge and were confined to cool habitats.
The weak dispersal power of mayflies also results in a high percentage of endemism. Many species colonizing cool running waters in the European Alps are found nowhere else, but have related species in the Pyrenees or the Carpathians. On some islands, such as Madagascar and New Caledonia, endemism in mayflies reaches 100%. In contrast, many species that are effective dispersers, especially in the baetid genus Cloeon, may have very wide distributions.
Worldwide, two families, the Leptophlebiidae and the Baetidae, are especially important both in terms of abundance and diversity, representing half of the known species. In contrast, the Siphlaenigmatidae (New Zealand) and Teloganellidae (Southeast Asia) encompass only one species apiece.
The distribution and abundance of mayflies has received considerable attention. Within the basic zoogeographical limitations, abiotic factors, notably temperature, substratum, water quality, and, in running water, current speed, seem to be the most important. Other factors, such as ice, floods, drought, food, and competition, may also influence abundance and distribution. Generally, the number of mayfly species decreases with increasing altitude.
Many lotic mayflies are either dorsoventrally flattened or streamlined as an adaptation to life in swift current. The physical substratum also traps different amounts of detritus and silt, and this is a major factor influencing microdistribution. The richest mayfly community is often found in association with aquatic vegetation, which, as well as providing shelter, functions as a detrital trap and as a substratum for periphyton. For burrowing mayflies, the presence of the correct substratum is obviously a major determinant of both macro-and microdistribution. In lakes, the highest mayfly diversity occurs in the shallow littoral areas. At deeper levels, the mayfly fauna, although often reaching high densities, is usually poor in species. Mayflies are generally absent from the profundal (the deep waters where light does not penetrate) of lakes. Many mayflies can tolerate a wide range of salinities, and a few species within the Baetidae, Caenidae, and Leptophlebiidae occur in brackish water.
Mayflies constitute a major part of the macroinvertebrate biomass and production in freshwater habitats. Seasonal variation in density, biomass, and annual production are strongly influenced by life cycle parameters, indicating the importance of correct life cycle information in production studies. Most mayfly production values, expressed in terms of dry weight per square meter per year, are in the range of 0.1-10.0g.
HUMAN INFLUENCE
Humans increasingly affect the distribution and abundance of mayflies and, by virtue of their widespread occurrence and importance in aquatic food webs and particularly in fish production, mayflies have been widely used as indicators of water quality. Baetis species are often among the most tolerant of mayflies to pollution. In North America, the use of mayflies as indicators of water quality has not escaped attention. The mass emergence of burrowing mayflies from Lake Erie and the Mississippi River has provided a useful barometer of water quality. Organic and nutrient enrichment of Lake Erie in the 1940s and 1950s led to an increase in the intensity and frequency of mass emergence of Hexagenia until 1953, when prolonged periods of oxygen depletion in the hypolimnion (the lower layer of cold water in lakes that stratify) caused the population to crash to virtual extinction. However, improvement of water quality has now led to a resurgence of emerging swarms. Pesticides also affect nontarget organisms such as mayflies, and Canadian studies in connection with blackfly control have demonstrated catastrophic drift and reduced biomass in mayfly populations over long distances in rivers treated with methoxychlor.
Acidification of freshwaters is a major threat to freshwater communities and many mayflies are affected adversely by low pH, and emergence is a particularly critical period. The genus Baetis seems to be particularly sensitive.
River and lake regulation (e.g., by impoundment in reservoirs) for water supply and power can have profound effects on the mayfly community, especially when there is a hypolimnion drain. For example, an increase in winter temperatures and a fall in summer temperatures may remove obligatory life cycle thresholds, leading to extinction. Fecundity may also be influenced by changes in water temperature. In reservoirs themselves, lentic (still water) conditions and increased water level fluctuations usually produce a reduced mayfly fauna, although there may be an increase in the abundance of burrowing and silt-dwelling species. The flooding of new areas can also create new habitats for mayflies, and in many of the large African reservoirs the mayfly Povilla adusta has developed large populations, which burrow into the submerged trees and play an important role in tree breakdown.
Deforestation is a major threat to mayfly biodiversity in the tropics, while in temperate regions loss of habitat and reduction in connectivity between freshwaters is becoming an increasingly important threat. In addition, climate change will affect many of the facets of mayfly biology and thus lead to significant changes in mayfly communities throughout the world.
