Ecdysis
Ecdysteroids
Specialized Terms
prothoracic gland A diffuse endocrine organ in the thoracic area of insects that produces ecdysone, the precursor of 20-hydroxyecdysone.
steroid A lipid containing a 17 carbon nucleus of fused rings consisting of 3 cyclohexane and 1 cyclopentane. ecdysone Synthesized by the prothoracic gland, it is the precursor to 20-hydroxyecdysone.
20-hydroxyecdysone The most common biologically active steroid in insects.
ecdysteroids Biologically active steroids related to ecdysone. juvenile hormones Terpenoid hormones that, in combination with ecdysteroids, orchestrate gene expression for larval stages of insects.
nuclear receptors A family of soluble proteins, which are mobilized by steroid hormones to coordinate gene expression through direct binding to DNA.
polytene chromosomes Giant chromosomes arising from replication without mitosis, allowing visualization of banding patterns and puffs indicative of transcriptional activity.
The ecdysteroids are steroid hormones that, in combination with juvenile hormones, program gene expression that is appropriate for each stage of insect development. Beginning at embryogenesis, ecdysteroid levels rise transiently during each stage to initiate molting. Depending on the level of juvenile hormone, elevated ecdysteroids mobilize several types of nuclear receptors that bind directly to DNA, either to promote or suppress gene expression. During the adult stage of many insects, ecdysteroids play important roles in reproduction, principally in the development of gametes.
DISCOVERY
Early in the 20th century scientists began to demonstrate roles for circulating hormones in the development and maturation of insects. Stefan Kopec, working in Poland, observed that removal of the brain in gypsy moths (Lymantria dispar), caused developmental arrest. Amazingly, reimplantation of the brain allowed development to resume. In other experiments, Kopec used an experimental tool called a “ligature” to demonstrate the timing of hormone release from the brain. A ligature is applied with a loop of string that is pulled tight, like a tourniquet, interrupting blood flow between front and back blood compartments. He found that if a ligature was tied around a last instar larva before the “critical period” of 5-7 days, the front part of the animal developed pupal cuticle, whereas development in the back part was arrested (Fig. 1). However, if the ligature was applied after the critical period, both sides developed. These observations implied that something was released from the front end of the animal during the interval between 5 and 7 days of development. Consistent with Kopec’s earlier experiments, this signal was later shown to come from the brain. These ingenious experiments illustrated the surprising fact that the insect brain, in addition to its well-known function in electrical signaling, is also a secretory organ, controlling developmental processes through release of hormones into the bloodstream.

FIGURE 1 Ligature tied prior to the critical period (arrow) leads to arrested development in the posterior part of the animal, where green .larval cuticle is retained. The thorax synthesizes a new, dark pupal cuticle in response to ecdysteroid release by the prothoracic glands
The experiments of Kopec were published in two classic papers in the early 1920s, but little happened for about a decade. The notion that hormones programmed insect development was not readily accepted, because thinking was dominated by studies of reproductive hormones in birds during the mid-19th century. These experiments showed that implantation of the male testes causes masculinizing effects, whereas removal of the testes produced loss of male characteristics. Such experiments performed in insects had no apparent effect, and as a consequence it became accepted that insects did not engage in hormonal signaling.
Nevertheless, Wigglesworth, S. Fukuda, and later Carroll Williams extended Kopec’s work, by providing evidence for a second signal located in the thorax of the insect that was released in response to the brain hormone. It became evident that implantation of the brain, as Kopec had done, worked only if the brain was placed in the thoracic area. When the brain was implanted into an isolated insect abdomen, developmental arrest persisted. The source of this second factor was the prothoracic gland. Williams showed that if both the brain and prothoracic glands were implanted into an isolated abdomen, development resumed. The prothoracic glands alone could accomplish this, provided that they had prior exposure to the brain. It therefore became evident that the brain provided a hormonal signal that induced release of a ” molting hormone ” from the pro-thoracic glands, which was critical to promoting growth.
CHEMICAL NATURE OF ECDYSTEROIDS
Attempts to isolate the molting hormone, or “ecdysone” as it came to be known, began in the 1940s and continued for the next 10 years, until the efforts of two German chemists, Butenandt and Karlson, yielded microgram quantities of pure ecdysone. Because of its water solubility, ecdysone at first was not recognized as a steroid. Soon X-ray studies of ecdysone crystals revealed it as a unique steroid with five hydroxyl substituents, accounting for its ability to dissolve in water (Fig. 2).
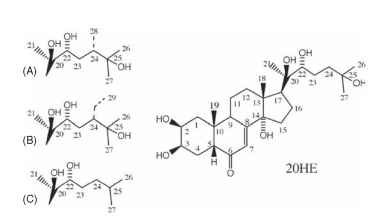
FIGURE 2 The structure of 20-hydroxyecdysone (20HE) and related ecdysteroids. The hydroxyl substituent of 20HE at position 20 confers biological activity to ecdysone. Many insects obtain “phy-toecdysones” from plants and convert them to biologically active forms. These molecules differ from 20HE only in the number of carbons in the alkyl side chain, shown for makisterone A (28 carbons; A), makisterone C (29 carbons; B). Ponasterone A (C), lacks the hydroxyl group at position 25.
The first ecdysone structure soon was recognized as a precursor to the biologically active material. Upon release from the prothoracic glands, ecdysone, or its 3-dehydro form in some insects, is converted to the active form upon arriving at target tissues. Those tissues capable of responding to the hormone produce the enzymes needed to attach a single hydroxyl group to the carbon at position 20, making it 20-hydroxyecdysone (20HE). This substance had already been identified in crustaceans as crustecdysone. The identical molecule functions as the biologically active hormone in both insects and crustaceans.
Not long after the identification of ecdysone, phytoecdysones were discovered in plants. The first, ponasterone A, was discovered by Koji Nakanishi and the identical molecule was later found in some crustaceans. This molecule, which differs from ecdysone in lacking a single hydroxyl at position 25, eventually proved useful in radiolabeled form for characterization of ecdysteroid receptors. Hundreds of phytoecdysones have been identified, including 20HE itself. Reasons for presence of phytoecdysones in plants are unclear, but they may serve defensive roles by disrupting the growth of herbivorous insects.
As more insects were examined, additional configurations of the basic ecdysone structure were found. This group of molecules now is collectively known as ecdysteroids. Insects, lacking the enzyme squalene synthase, have a dietary requirement for cholesterol in order to synthesize ecdysteroids, which occurs via a series of synthetic steps involving cytochrome P450 enzymes. These enzymes are encoded by a family of “Halloween” genes (e.g., phantom, spook, disembodied, shadow, shade) that have been identified as mutant phenotypes in the fruit fly Drosophila. In many instances, phytosterols such as campes-terol and (3-stigmosterol are converted to hormonally active ecdyster-oids makisterone A and makisterone C, respectively (Fig. 2).
SOURCES AND FUNCTIONS FOR ECDYSTEROIDS
During the immature stages of development, the chief source of ecdysteroids is the prothoracic gland, a diffuse organ located in the thorax. In higher insects such as Diptera and Hymenoptera, the pro-thoracic gland has become part of a composite structure called the ring gland. The precursors ecdysone or 3-dehydroecdysone are synthesized and released into the blood. Conversion to 20HE occurs in target tissues such as epidermal cells, salivary glands, fat body, nervous system, gut, and imaginal discs by 20-hydroxymonooxygenase.
During each stage of development, feeding and growth are followed by a sudden elevation of ecdysteroids, which induces animals to stop feeding and to engage in a new round of gene expression appropriate for the next stage. The epidermal cells begin to secrete a new layer of cuticle and to take back most of the old cuticle, recycling the chitin and protein recovered into the new layer. If the next stage is to be larval, ecdysteroids circulate with high levels of juvenile hormones, and a new set of larval characters is expressed. However, when larval development is complete, metamorphosis is signaled by short ecdys-teroid pulses in the absence of juvenile hormones. Completely new structures are created as the insect undergoes the process of changing from a caterpillar into a reproductive, winged adult.
The actions of ecdysteroids can be observed almost immediately after their release as “puffs” on large polytene chromosomes in the salivary glands of some flies. Even before ecdysteroids were chemically identified, Clever and Karlson noticed puffs within hours of injecting the natural hormone and suggested that it acted to regulate gene expression. This was very insightful, because it is now known that ecdysteroids have direct actions to either activate or suppress the expression of many genes via nuclear receptors, and that the puffs correspond to gene loci at which transcription takes place.
A model explaining relationships between temporally distinct puffs was proposed by Michael Ashburner in the early 1970s. He found that just after ecdysteroid release, a set of early puffs could be observed, followed by early-late and late puffs. With the identification of ecdysteroid receptors, his model now views early and early-late puffs as evidence of transcriptional activity induced by EcRs and other nuclear receptors. The gene products resulting from these events then give rise to late puffs and also repress further transcriptional activity at early puffs. In this way, a complex but coordinated series of gene expression events occurs, initiated by EcRs.
Having been present throughout larval development, the pro-thoracic glands degenerate during metamorphosis. Nevertheless, ecdysteroids persist in the adult stage, where they play important reproductive functions. The source of ecdysteroids in adults remained a mystery for many years, although anecdotal accounts implicated the mobile oenocytes as a possibility. In the 1970s, Henry Hagadorn provided a breakthrough, showing that mosquito ovaries produce large quantities of 20HE, and that the hormone is required for vitellogenesis, or yolk deposition in developing oocytes. The precise source of ecdysteroids is the follicle cell layer surrounding the oocyte. Since Hagadorn’s initial finding, it has been shown that ecdysteroids are produced in sheath cells in the testes, where they are involved in sperm maturation.
MOLECULAR BASIS OF ECDYSTEROID SIGNALING
Ecdysteroids belong to a large class of steroid signaling molecules that enter all cells in the body. Because of their lipophilic character, they pass through the cell membrane easily. Whether they affect the cell upon entry depends on the presence or absence of specialized proteins belonging to a large class of soluble, diffusible nuclear receptors. Nuclear receptors get their name from that part of the cell in which they conduct their business, which is the regulation of gene expression. Early in the 1990s, David Hogness and colleagues discovered a class of nuclear receptors in fruit flies they called “ecdysteroid receptors” (EcRs), which are distant relatives of the vertebrate farnesol X receptor. Upon their activation by ecdysone binding, EcRs bind directly to DNA at “ecdysone response elements” (EcREs) to turn genes on or off. Further work showed that, in order to bind with high affinity to DNA, the EcR first finds a partner protein to form a doublet, or “dimer” complex. The most well-known EcR partner is “ultraspiracle” (USP), an ortholog of the vertebrate retinoid X receptor. It is this protein dimer that, together with co-activator proteins, binds with high affinity to EcREs, resulting in regulation of gene expression.
It is well known that EcRs have many different types of effects on cells, causing some to differentiate into muscles, glands, or to form particular kinds of cuticle and cuticular structures appropriate for a larva, pupa, or adult. How is this diversity of effects encoded by ecdys-teroid receptors? It is a very complicated process, and many questions are under current investigation. But it is known already that several different types of ecdysteroid receptors occur in insects, including EcRA, EcRB1, and EcRB2. These receptor ” subtypes ” occur at different stages of development and can be specific to particular tissues. For example, the A isoform is expressed in tissues that change during metamorphosis, including imaginal discs, ring gland, and neurons that undergo programmed cell death after adult emergence. The B1 isoform is expressed in larval tissues such as the salivary gland, fat body, and larval muscle. Thus ecdysteroids may specify stage-specific phenotypes by signaling through this diversity of receptor subtypes. Another point is that the partners with which they bind to DNA could vary substantially, providing a further level of combinatorial diversity.
ECDYSONE-BASED INSECT CONTROL
One way of managing pest insect populations is to target unique aspects of their physiology. Because molting is a particularly unique aspect of insect biology, scientists have attempted to learn more about their hormonal control systems, so as to design ” magic bullets ” targeting only insects. The first complete synthesis of ecdysone was accomplished by John Siddall in the late 1960s, but development of such a complicated molecule has not proven to be commercially viable. As insecticides go, the ecdysone structure itself is rather complex and would be expensive to produce on a large scale; moreover, it is too unstable in the environment to have any usefulness in field applications.
Nevertheless, chemicals with unexpected biological activities are produced every year by the chemical industry, and it has become routine to test these compounds using all available biological assays. This has led to unexpected successes on many occasions, including the serendipitous discovery of the first ecdysone agonists or “ecdy-sanoids” by the Rohm and Haas Company in the 1980s. Keith Wing and colleagues at Rohm and Haas described a series of bisacylhydra-zines that have astounding ecdysone-like activity, even though their structures were not recognizable as steroid-like. Application of these compounds causes premature molting, aberrant differentiation of cells, and death of insects due to improper programming of development. The ecdysanoids have been commercially developed for agricultural pest control and provide an encouraging example of how targeting basic insect physiological processes can lead to safer and more environmentally sound insect control agents ( Fig. 3 ).
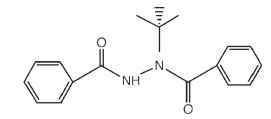
FIGURE 3 The structure of RH5849, one of the first ecdysone agonists developed by the Rohm and Haas Company.

