Insect collecting often begins in youth, when one discovers the love of making specimens for school, scouts, 4-H clubs, and other projects or as a fascinating pastime in its own right. The great diversity and numbers of insects, plus their rapid life cycles, usually mean insect populations can afford to give up some of their numbers and not be adversely affected by most collecting activities.
As one becomes engaged in various facets of insect biology as a researcher, the collection of specimens is important for taxonomic research, ecological studies, bioassessment and biomonitoring, and physiological and genetic studies. Because each labeled specimen is a historical record of that species’ occurrence in time and place, proper methods of collecting, preparing, labeling, and storing are vital.
The general habitats, collecting equipment needs, and methods of collection and storage for the major insect orders and order groupings are presented in Table I. Below, a description of each type of equipment and its use are given. For more extensive illustrations and descriptions consult the topics listed under Further Reading.
BASIC EQUIPMENT FOR
COLLECTING INSECTS
1. Aerial net—A net bag made of translucent netting so one can see what’s inside; it can be used as a beating net if needed. The net is used to grab insects off plants or to cover them on the ground. Since insects tend to crawl or fly upward, hold the net so they move toward its closed end once they are inside.
2. Beating (or sweeping) net—A heavy cloth bag, perhaps with small netted area at the bottom; it is used to sweep “like a broom” through vegetation many times. To use, strongly wave the net to concentrate insects in bottom of the net before placing net with insects into a killing jar until movement ceases. Then pick out what is desired and allow the rest to revive and go free.
3. Aquatic net—A heavy-duty metal hoop that can be D-shaped or round supports the netting. The former type is best for stream bottoms. The mesh and heavy cloth skirt have to be strong enough to take a beating. To use, hold the net against the bottom of the stream riffle and disturb the substrate upstream to allow insects to flow into net, or “work” the net among plants or debris to catch pond insects.
4. Malaise trap—A tent-like structure made of netting and designed to direct insects that encounter it to climb upward and follow the seams to a collecting container into which they fall. Container can be designed for live capture or killing in alcohol or by means of a dry poison such as cyanide powder.
5. Lights and light traps —A battery-powered light bulb such as a 15 W fluorescent “black light” or self-ballasted mercury vapor lamp can be hung from a tree limb or other support about one-half meter in front of a white sheet strung between two trees in the forest. The collector then picks the desired insects off the sheet. Various trap designs are available from supply houses, in which lights attract the insects that hit one of four vanes (or baffles) surrounding the bulb and above a funnel, into which the insects fall when they hit a vane. Ethyl acetate in tins with “wicks” of cloth provide a killing agent; crumpled paper also can be used in the bucket below for live capture.
6. Pitfall traps—Tin cans, jars, or pails can be placed in holes dug in the ground and filled with earth to the outside rims. One may bait with dead animal matter or other attractants. Ethylene glycol (antifreeze) is often used as a killing agent. Walls of boards can also be erected narrowing to the opening of the pitfall to direct arthropods to the pit.
7. Beating sheet—A square of bedsheet or similar white cloth placed under a bush or tree to catch insects when they are knocked off after the plants are struck with a large stick, such as an axe handle. Insects are then collected by aspirator or forceps.
8. Aspirator—A tube plugged with a rubber cork in which are inserted two tubes: one bent and used to point at tiny insects; the other connected to a rubber tube for inhaling quickly to suck the insect into the tube. The latter one has a tiny screen attached to the inside end to prevent insects from getting into one’s mouth.
9. Berlese funnel—A commercial funnel of any size is needed, equipped with a screen inserted just above the narrow spout to prevent material from falling out. Leaf litter, birds’ nests, and other organic matter are put into the funnel, which is mounted on a rack or ring stand. A light bulb is placed over the top to dry out the organic material, driving arthropods downward as they seek moisture. Insects then fall through the screen and into a jar of 70% alcohol placed under the spout. The Berlese funnel is left in place until the organic matter is completely dried out.
10. Relaxing box—A tight container (plastic refrigerator boxes are excellent) is chosen in a size needed. Cut or fold paper toweling to line the bottom of the box at least 1 cm deep. Moisten the paper thoroughly with water, but leave no water standing. Add a small amount of an anti-mold chemical such as paradichloroben-zene or carbolic acid (phenol). Place a piece of stiff cardboard above the wet paper as a platform for the specimens. Freshly killed insects, or dried ones you wish to pin or spread, can be softened in the box. If left in the “relaxer” too long, however, they may mold or turn mushy and disintegrate.
11. Killing jar—A glass or plastic jar of desired size can be made into a killing jar by putting about a 1 cm layer of plaster of Paris in the bottom, or use just a pad of absorbent material such as cellucot-ton, cotton, or soft tissue. A fluid killing agent such as ethyl acetate or fingernail polish remover containing acetone is added to be absorbed by the plaster or other material. Be sure not to have any fluid on the walls of the jar, or specimens will be spoiled. If you use cotton or other absorbent material, cut a cardboard disk to separate the insects from the pad of killing agent.
METHODS
Insects are prepared for study and storage in three basic ways: pinning, fluid storage, and mounting on microslides. Adult insects or the immature forms of hard-bodied insects such as those with incomplete metamorphosis are pinned through the thorax of the body, unless too tiny, and then they are mounted on card points (see Section Placing Insects on Card Points). Insect pins, available from supply houses, are long and very sharp. They range from tiny headless ” minuten nadeln ” for mounting specimens on tiny blocks of foam, which in turn are put on regular insect pins, to pins that are numbered to match the general size of the insect. Size 000 is the smallest made and bends very easily. Most small insects that can be pinned are at least 5 mm in length, with a thorax big enough to hold the pin. Most medium and large insects are pinned on sizes 1 to 3. Sizes 4 to 7 are sometimes available for large specimens.
Preserving Insect Specimens in Fluid
Insects that are too small, or the bodies of which are too brittle or soft, should not be pinned. They should be stored in glass vials in 70% ethyl alcohol (EtOH). Other special fluids, especially those that preserve colors, can be learned from the works under Further Reading. Actually collecting in alcohol can be done using traps of any type (light, malaise, pitfall, and some bait traps). The larger insects can be dried out later and pinned. However, collecting in fluid is not recommended for collecting Lepidoptera (butterflies and
TABLE ICollection and Preservation of Insect Specimens for Insect Orders |
||||
| Taxon | Habitat | Equipment to use | Collection method | Preparation |
| Protura, Diplura, and | Leaf litter, rotten logs and stumps, | Berlese funnel, aspirator, wet | Place in funnel for several days, jar of alco- | 70% EtOH, mount on microslides |
| Collembola | birds’ nests, other detritus | brush | hol beneath, light above | |
| Thysanura and | Buildings (silverfish), leaflitter, | Forceps, Berlese funnel | Same as above | 70% EtOH |
| Microcoryphia | logs, seashores | |||
| Ephemeroptera | Naiads: streams, rivers, lakes | Dip nets, grab samplers | Kick samples, pick off stones | 70% EtOH |
| Adults: fields and forests | Aerial nets, light traps | Pick off plants or from light sheet | ||
| Odonata | Naiads: streams, lakes, ponds | Dip nets | Dredge or kick sample with net | 70% EtOH, place in envelope, wings |
| folded over back, and card with | ||||
| collecting data; spread for display | ||||
| Adults: fields, near streams and | Aerial nets | Sweep fast from behind with net | ||
| ponds | ||||
| Plecoptera | Naiads: streams | Aquatic nets | Kick-netting in riffles, pick off stones, sweep | 70% EtOH |
| shore vegetation | ||||
| Adults: along streams at lights | Light trap, aerial and sweep | |||
| nets, light trapping | ||||
| Orthoptera and other | Fields, forests, gardens, and other | Sweep nets, light traps, aerial | Sweep and aerial netting, light trap | Mount on insect pins, support body |
| orthopteroids | terrestrial habitats | nets, hand capture | sampling | until dry |
| Hemiptera, Homoptera, | All terrestrial habitats | Sweep nets, beating sheet, | Sweep and aerial netting light trap sampling | Pin large bugs, small ones on card |
| vegetation and other | examine plants, light traps | points or store in 70% EtOH, | ||
| hemipteroids | scales on microslides | |||
| Phthiraptera | Avian and mammalian hosts | Forceps, aspirator | Scrape fur and feathers | 70% EtOH, mount on microslides |
| Thysanoptera | Plant axils, flower parts, and other | Aspirator | Examine plants and aspirate | 70% EtOH, mount on microslides |
| plant parts | ||||
| Neuroptera and | Larvae aquatic (mostly streams) | Aquatic nets, sweep nets, and | Kick sampling in riffles, sweep vegetation, | 70% EtOH or pin |
| Megaloptera | or on plants | light traps | examine trap samples | |
| Coleoptera | All habitats | Aquatic, aerial, and sweep nets: | Bait pitfall traps with rotting animal flesh, | Pin or mount on card points |
| light, malaise and pitfall traps | other methods as above | |||
| Mecoptera | Woodland glades, understories | Sweep and aerial nets, light trap | Follow and net individuals seen, use light | Pin or place in 70% EtOH |
| trap (Meropeidae) | ||||
| Lepidoptera | All habitats, esp. fields and woods | Aerial net, sweep net, bait, | Net resting butterflies, bait traps with rot- | Relax, and then spread on spreading |
| malaise, and light traps | ting animal flesh and excrement or fer- | boards, use 70% EtOH or special | ||
| menting fruit, sweep or examine plants | fluids for larvae | |||
| for larvae | ||||
| Trichoptera | Running water, esp. streams for | Aquatic, sweeping, and aerial | Kick samples for some larvae, others must | Store all stages in 70% EtOH |
| larvae | nets | be picked in cases off rocks in stream | ||
| Adults may be near or far from | Malaise and light traps | Adults come to lights or can be swept from | ||
| breeding sites | streamside vegetation | |||
| Diptera | All habitats; larvae most common | All kinds of nets, dippers, light | Examine plant and animal hosts, capture in | Pin, place on card points, or store in |
| in aquatic or moist habitats in | traps, malaise traps | net, traps | 70% EtOH | |
| water and land or animal hosts | ||||
| Siphonaptera | Bodies and nests of birds and | Aspirator or moistened brush, | Comb animal, break up nest over white | Place in 70% EtOH, mount on |
| mammals | sweep net | background, sweep grassy areas around | microslides | |
| infested buildings | ||||
| Hymenoptera | All terrestrial habitats | Nets, all trap types | Collect from flowers, sweep, extract from | Mount on pins; on card points or in |
| light, malaise, pitfall, and other traps | 70% EtOH if small | |||
moths) or Culicidae (mosquitoes) because they have patterns formed of colored scales and those may be ruined by the fluid.
Vials used are often of the “patent lip” type with neoprene stoppers. The author prefers to use 4-dram vials with size 0 stoppers and store them in plastic racks and cardboard boxes with partitions available from supply houses. A better alternative is the screw-cap vial, which should be equipped with “polyseal” plastic sealing inserts. One of the biggest problems with liquid-stored specimens is the drying out of the fluid. I believe the latter storage to be superior because the alcohol does not discolor with years nor does the cap change shape (stoppers swell or stick to the glass).
Many tiny insects such as lice, fleas, and thrips can be stored in EtOH until such time as they can be made into permanent micro-slide mounts with Euparol, Canada balsam, or some other mounting medium.
Pinning Insect Specimens
1. Be sure the insects to be pinned are soft enough so that they will not crumble when you handle them and attempt to pierce them with the pin. These can be just-caught, or they can be softened, if dry, in a relaxing box (see earlier).
2. Select the pin and pierce the high point of the thorax with the point. Push the pin straight through the thorax. Check straight-ness by observing from front and side to see if the pin is perfectly perpendicular to both the transverse and the longitudinal axis of the insect.
3. When the insect, such as a grasshopper, has a middorsal ridge in the thorax, pin just to the right of the ridge.
4. For beetles, insert the pin in the right elytron (front wing) close to the midline. Do not pin beetles through the prothorax.
5. Push the pin on through when you are satisfied with the position. One-third to one-fourth of the pin should be showing above the insect’s thorax.
6. If the abdomen or legs are drooping, push the pinned insect into a block of foam plastic or a cardboard box to support these parts until they are dry. Then remove the insect and label it.
7. Most museum specimens do not have legs and antennae adjusted to a life-like position when they are pinned. However, for display purposes or personal satisfaction one may move these body parts into desired positions on the foam or cardboard support and fix them temporarily with pins over or against them.
Placing Insects on Card Points
A card point is a small wedge of high-quality (100% cotton content) cardstock, punched from the sheet with a special punch obtainable from a supply house. There are several different shapes, but the author prefers the ones with the wide end rounded.
Card pointing is used for tiny insects that are hard-bodied enough not to lose shape when dried. Size usually ranges from 1 to 5 mm or slightly larger in length. The author normally selects from large samples of dried specimens collected in sweep samples or light traps.
1. Punch out a number of card points. Place them on top of a firm foam plastic or cardboard surface.
2. Push the point of an insect pin into the wide end a short way from the very end, and push the card point up the pin by inserting the pin with the card point into the top hole of a three-step pinning block (wooden block with three fine holes of different depths to provide uniform heights of labels on pins) and pushing the point
up until it stops. It should be about one-third the distance from the top of the pin.
3. Use forceps to turn the very tip of the card point downward at a right angle to form a vertical surface.
4. Put a tiny dab of glue on the vertical surface you have made with the forceps. When doing a number of specimens, put a small drop of glue on a piece of card or paper to use (although it will tend to harden on the surface after a minute or two).
5. Position the insect so that the right side of the thorax is accessible, and touch the glue-covered surface of the card point to the right side of the thorax. (The insect should appear to be “holding onto the card point with its right hand.”) Use forceps to position the insect firmly against the glued surface and have it positioned so that its orientation to the ground is as it would be in life.
6. Fill out your insect label with locality, date, and collector’s name. Trim it to be as small as possible (avoid large, oversized “barn door” labels). Labels should be printed on 100% cotton light card stock in permanent black (India) ink or can be done on a postscript laser printer.
7. Position the label on the pin and push it up the pin at the middle hole of the three-step pinning block. As you read the label, the card point and insect should be projecting to the left of the pin shaft. Make sure both card point and label are not tilted or crooked.
8. Place the specimen in a temporary holding unit tray until it can be identified and put in the collection. Identification labels should be affixed below the collecting data label and in a position so that both labels can be read from the same angle. The lowest step on the three-step pinning block is normally used for the identification
label.
INSTRUCTIONS FOR SPREADING BUTTERFLIES AND MOTHS
1. Have all needed items ready: well-softened specimens (stored in relaxing box or freezer after collecting), spreading boards of proper sizes, straight strips of tracing or waxed paper or other material, setting needles or picks, insect pins for specimens, glass-headed pins for holding paper in place (insect or dressmaker’s pins okay). See Fig. 1 .
2. Fix paper strips along the side boards of spreading board, slightly back from the notch to allow you to work the wings into place. Use two or three pins at the top of board to hold the paper even down its length.
3. Push a proper sized insect pin straight down through the thorax of specimen, so it is not tilted in any direction. Push the pin far enough that the top of the thorax is one-third to one-fourth the distance down from the pin head (Fig. 1A).
4. Push the pin down into the soft material in the notch of the spreading board so that it is not tilted in any direction. Also, push it far enough that the wings, when out straight to the side of the insect, rest flat on the side boards of the spreading board. Be sure you do not place the insect too close to the top of the board (leave room to pull wings into proper position).
5. Push an insect pin down along the left rear of the thorax, behind the base of the left hind wing, to keep the body from swinging left as you position the wings.
6. Place paper over the wings. Hold the left-hand paper strip in the thumb and forefinger of your left hand while you now begin to position the wings.
7. Insert a sharp insect pin or setting pick behind the costa vein close to the base of the left forewing. Swing that wing upward until the inner (anal) margin is at a right angle to the plane of the body (Fig. 1B). Be sure not to let the hind wing pop out from below the forewing. Insert a glass-headed pin into the paper above the costa near the base and inner margin near the anal angle to hold the wing secure.
8. Pull the left hind wing forward by inserting a setting pin or pick behind the radial vein near the wing base, and swinging it forward. Leave a small triangular space between the outer margin of the hind wing and the inner margin of the forewing. Fasten paper over the left hind wing by putting a pin below it near the wing base.
9. Repeat procedures 6 to 8 on the right side, and be sure you have produced symmetrical results (Fig. 1C ).
10. Position the antennae with pins to look as shown in Fig. 1 . The abdomen may need to be supported with crossed pins beneath it or held down straight with crossed pins above it.
11. Write data (where, when, and by whom collected) on the paper strip holding down the wing or make a label and tuck it under the paper strip until the specimen is taken off the board (Fig.
1D ).
12. Add other specimens below, as close together as you can, if you have many specimens to spread.
13. Make a notation of the date of spreading on the paper strip to remind you of how long the specimens have been on the boards.
14. Store the board in a pest-free, dry place such as a steel or wood cabinet. Fumigation of the storage enclosure is recommended.
15. Allow specimens to dry for at least a week, longer if possible. If the abdomen is completely dry and stiff, the specimen should be ready to remove.
STORAGE OF SPECIMENS
Specimens that you would normally pin or spread after pinning can be placed in envelopes. This is known as “papering.” Glassine stamp envelopes are excellent, but any kind will do. To make triangular paper envelopes, cut rectangles of paper, one side about a half-inch longer than the other. Fold into a triangle and then fold down the remaining “flaps” after putting the insects inside. Be sure butterflies and moths have wings folded over their backs for best results. They can be softened in the relaxing box at any later time. Don’t forget to put collection data on the envelope.
Storage of pinned and papered specimens must be in tight containers so that museum pests such as Dermestidae (carpet beetles) and topiclice (Psocoptera) cannot get to them. These can also be repelled by fumigants such as naphthalene (moth flakes or moth balls), PDB (paradichlorobenzene), or dichlorvos-impregnated ” strips ” cut into blocks. However, the trend is away from museum fumigants because of possible health problems from exposure to them. The better method is freezing. Whole boxes can be left in a freezer for a few days on an annual basis to kill any pests that may have entered.
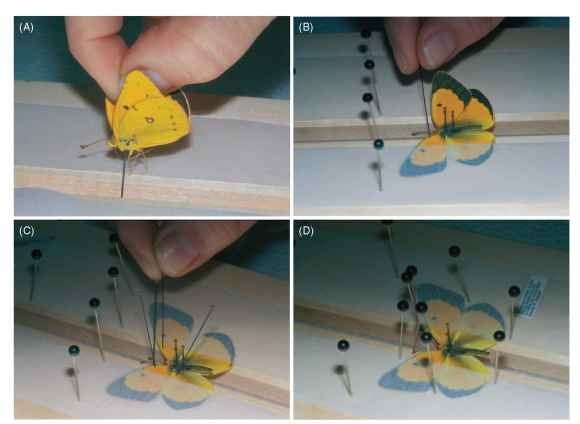
FIGURE 1 (A) Orange sulfur butterfly (Colias eurytheme) with insect pin inserted at proper height, ready to place in groove of spreading board. (B) Insect pin inserted behind thick costa margin and pulled forward so that inner margin of forewing is at right angle to groove. (C) After left hind wing is pulled forward and secured, right forewing and hind wing positioned to match left. (D) Glass-headed pins in proper position to hold tracing paper tight for at least 1 week, until the insect dries and can be removed; label ready to add.
Drawers and boxes housing pinned specimens must have tight-fitting lids with inner flanges higher than the outer walls of the unit. Thus, a tight seal can be achieved, which usually keeps pests out. Equipment dealers offer high-quality “Schmitt” boxes and standard cabinet drawers of different dimensions (Cornell, U.S. National Museum, and California Academy types are most common), as well as cabinets to house them. Homemade boxes and cigar boxes will do in a pinch; just add a foam plastic lining. However, one cannot expect such boxes to be pest-proof without fumigation.
Vials with alcohol-preserved specimens and microscope slides can be stored in special boxes or cabinets also available from dealers or built yourself.
