Insects have an open circulatory system. This means that the internal organs and tissues are bathed in hemolymph, which is propelled actively to all internal surfaces by specialized pumps, pressure pulses, and body movements and is directed by vessels, tubes, and diaphragms (Fig. 1). Without such constant bathing, tissues would die. The internal organs and tissues depend on the circulatory system for the delivery of nutrients, and to carry away excretion products, and as the pathway by which hormone messengers coordinate development and other processes.
Gas exchange in insects occurs via the tracheal system, which supplies all internal organs with tracheole tubules from spiracular openings in the body wall of terrestrial insects or from gill structures in

FIGURE 1 Delivery of the hemolymph to all tissues is so vital that a number of structures have evolved to ensure complete circulation. Principal circulatory organ is the dorsal vessel which is supported by the underlying dorsal diaphragm and the ventral diaphragm (omitted in the diagram). Circulation in the appendages is effectuated by accessory pulsatile organs (modified after Pass 2000).
aquatic insects. However, the hemolymph has the capacity to dissolve carbon dioxide gas in the form of bicarbonate ions. A few insects live in low oxygen environments and have a type of hemoglobin that binds oxygen at very low partial pressures, but for the most part oxygen is supplied and carbon dioxide is removed by ventilation through the tracheal system. Besides the functions already mentioned, the circulatory system provides a medium in which battles are fought between the insect host and a myriad of invading disease microorganisms, including viruses, bacteria, fungi, and insect parasites. Principal participants in these interactions are the blood cells or hemocytes.
While maintaining the body tissues, the circulatory system is the medium in which homeostasis is ensured, including the regulation of pH and inorganic ions, as well as the maintenance of proper levels of amino acids, proteins, nucleic acids, carbohydrates, and lipids. Any change in the hemolymph quickly affects all organs bathed. The time for complete mixing of the hemolymph depends on the size of the insect, but it can be up to 5 min in a resting adult cockroach weighing about a gram. Any substance injected into a healthy insect will eventually appear at the extreme ends of all appendages in a few minutes, emphasizing the efficiency of the delivery mechanisms, which can be marvels of microhydraulic engineering.
DORSAL VESSEL
The principal organ of hemolymph propulsion is the dorsal vessel, or at least it is the most visible organ specialized in hemolymph movement in insects. It forms a hollow tube which runs along the midline for the whole length of the body. Contraction of its circular musculature results in a contractile stroke (called systole), whereas elastic connective tissue strands, which connect the dorsal vessel to the body wall, are responsible for dilation and opening of the vessel (called diastole).
The dorsal vessel of most insects is not uniform in its course through the body and shows a differentiation into two regions which is reflected by their traditional denomination: the posterior part in the abdomen is referred to as the “heart,” whereas the anterior part in the thorax and the head is the “aorta.” Both terms are borrowed from better-known vertebrate structures and give an inaccurate impression of the different roles of those structures in insects. In phylogenetically ancestral insects, the flow of hemolymph in the dorsal vessel is bidirectional: upon contraction hemolymph flows toward the head in the anterior part and simultaneously toward the rear end in the posterior part of the dorsal vessel (Fig. 2). The posterior directed flow is caused by an intracardiac valve in the abdomen and supplies the long caudal appendages (cerci and terminal filum), for example in some apterygotes and mayflies. However, in most insects the hemolymph flow is unidirectional in that the contraction of the dorsal vessel begins at the posterior end and advances forward as a peristaltic wave. In the pupae and adults of Coleoptera, Diptera, and Lepidoptera there is a regular change in the direction of the contraction wave, termed heartbeat reversal. Contraction waves of the dorsal vessel toward the head alternate with waves toward the rear of the body; between these contractions are short phases of rest.
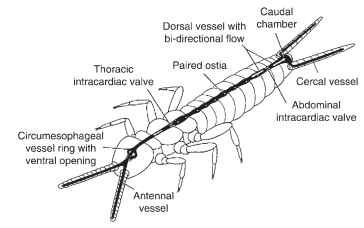
FIGURE 2 In the primitive insect Campodea (Diplura) the dorsal vessel exhibits a bidirectional flow. This enables the supply of the antennae and the cercal appendages by vessels connected to the dorsal vessel.
Hemolymph flows into the dorsal vessel through a pair of lateral openings (called ostia) in each abdominal segment. Valves which project into the vessel lumen close the ostia at the systolic contraction. Hemolymph emerges from the dorsal vessel at the open anterior end, and in insects with heartbeat reversal it usually also flows out the open posterior end. In Lepidoptera, there are two-way ostia which permit influx during forward phases and outflow during backward phases. The anterior end of the dorsal vessel opens just beneath or in front of the brain. This arrangement ensures a constant supply of nutrients and removal of waste products to and from the brain mass. In addition, the dorsal vessel is often intimately associated with the retrocerebral nervous system (including the hypocerebral ganglion, corpora cardiaca, and corpora allata complex) just behind the brain, which delivers neu-rohormones and possibly other hormones into the aorta at specialized release sites. Some insects have in addition outflow openings which are paired or unpaired and located more ventrally. In cockroaches, man-tids, and some orthopterans, the outflow openings are outfitted with sphincter-like valves and associated with segmental vessels laterally diverging from the heart. These vessels are formations of connective tissue with no inherent musculature, thus providing a simple channel to ensure lateral perfusion of the pericardial sinus.
The dorsal vessel is comprised of two rows of opposing pairs of muscle cells (collectively called myocardium). The cells of each row are offset, so that they cause a spiral-like peristaltic wave of contraction. The myocardium in all insects is spontaneously active, usually beginning in the embryonic stages. This type of heart control is termed myogenic, because the electrical activity underlying contractions arises in the myocardium itself. This is in contrast to a neuro-genic heart control present in crustaceans, such as crabs and lobsters, in which a barrage of nervous impulses drives the heartbeat from a discrete cardiac ganglion center. In insects, the myogenic pacemaker may be neurally or hormonally modulated. The basal heartbeat rate of most insects is around 60 beats min-1 at room temperature and at rest (e.g., in the American cockroach, Periplaneta americana, and the locust, Locusta migratoria). In adult house flies (Musca domes-tica), however, it ranges between 300 or more beats per minute during flight to zero beats for a while when at rest. The central nervous system of the adult house fly is composed of the brain and a thoracic ganglion mass, but lacks abdominal ganglia. Because of this unusual anatomy, the dorsal vessel of the abdomen can be separated from central nervous system input by experimentally severing the thorax from the abdomen. After this operation, the heartbeat of the fly becomes quite regular at around 60 beats min- 1. This indicates that the heart of the house fly is innervated by both inhibitory and excitatory motor neurons from the central nervous system.
DORSAL DIAPHRAGM
The dorsal diaphragm is a fenestrated membrane which separates the upper pericardial sinus from the lower perivisceral sinus. It consists of connective tissue with associated muscles which are called alary muscles because in some insects, for example cockroaches, they resemble wings projecting laterally from the dorsal vessel of each abdominal segment. Although sometimes mistakenly thought to play a key role in heartbeat, the alary muscles are more properly called muscles of the dorsal diaphragm. Whereas the myocardium is specialized to contract rapidly and constantly, the ultrastructure of the alary muscles indicates infrequent and slow contractions. The muscles associated with the dorsal diaphragm may likewise be arranged in a loose network, as in Lepidoptera, or arranged like a weave surrounding the dorsal vessel, as in some Diptera; the functional role of these muscles is difficult to determine.
VENTRAL DIAPHRAGM
The ventral diaphragm plays a prominent role in perfusing the ventral nerve cord of insects. Nearly 40 years ago Glenn Richards surveyed the ventral diaphragms in insects and found that insects with a well-defined ventral nerve cord in the abdomen also have a well-developed ventral diaphragm. In contrast, insects with the ventral nerve cord condensed into a complex ganglion structure in the thorax invariably lack a ventral diaphragm. This correlation suggests that the role of the ventral diaphragm is inexorably tied to perfusion of the ventral nerve cord in the abdomen. When present, the ventral diaphragm loosely defines a perineural sinus below and the perivis-ceral sinus above containing the gut. In some insects, the ventral diaphragm is a strong muscular structure with a great deal of contractile activity. The activity of the ventral diaphragm is dictated by innerva-tion from the central nervous system. In some large flying insects, the ventral diaphragm assists in hemolymph flow during thermoreg-ulation by facilitating the removal of warm hemolymph from the hot thoracic muscles to the abdomen for cooling. The intimate association between the ventral diaphragm in insects and perfusion of the ventral nerve cord is strengthened by considering the structure in cockroaches that takes the place of a proper diaphragm. In these insects, four stripes of muscle, together called hyperneural muscle,are near the back of each of the abdominal ganglia, and contract slowly but not in a rhythmic order. The muscles are electrically inex-citable, which means that they do not contract myogenically, as the myocardium does, but instead are neurally driven by motor neurons located in the ventral ganglia. Thus each of the ventral nerve cords in cockroaches has its own muscle supply that pulls it back and forth along the midline of the abdomen upon demand thereby increasing the contact and mixing between the ganglia and the hemolymph.
ACCESSORY PULSATILE ORGANS
The dorsal vessel and the two large diaphragms are responsible for pumping hemolymph through the main body cavity. However, these organs are incapable of achieving circulation in body appendages such as the antennae, legs, wings, and various long abdominal processes (e.g., cerci and ovipositors). To supply these appendages, insects rely on special, small circulatory pumps, known collectively as accessory pulsatile organs or accessory hearts. As a rule, accessory hearts are separate from the dorsal vessel and function autonomously. The pumping organ is generally located at the base of the appendage and is connected to vessels or special diaphragms which guide the flow of hemolymph through the appendage. Accessory pulsatile organs are present in an astounding array of functional constructions. They are evolutionary novelties of higher insects and are absent in the phylogenetically ancestral insects. Antennal circulatory organs are nearly universal in insects, lacking only in groups with extremely short antennae, such as fleas and lice. The ancestral state of antennal circulation in insects is antennal vessels connected to the dorsal vessel as in certain apterygotes (Fig. 2). The connection between the two vessels was probably lost during the further evolution of insects and replaced by autonomous pulsatile organs at the base of the antennal vessels. The anatomy of these organs differs widely in various insects. The best investigated antennal heart in terms of morphology and physiology is that of the American cockroach (Fig. 3 ). Remarkably, the antennal heart of the cockroach functions not only as a circulatory pump but also as a neurohemal organ: hormones released into the ampulla lumen are pumped into the antennae where they most likely modulate the sensitivity of the numerous sensilla.
In leg circulatory organs, the flow of hemolymph is guided by longitudinal diaphragms instead of vessels. A diaphragm of connective tissue divides the inner cavity of each leg into two channels permitting a counter-current flow. Some insects have pulsatile leg organs with muscular attachment to the diaphragm. When the muscle contracts, one channel is compressed forcing hemolymph toward the thorax; at the same time, the other channel expands drawing hemolymph into the leg (Fig. 1). Other insects utilize changes in the volume of an elastic tracheal sac in the legs as the driving force for hemolymph exchange. A common misconception is that insect wings are dead cuticular structures. However, the veins of the wings are filled with hemolymph to maintain living tissues, such as nerves and tracheae. Circulation is achieved by pumping organs in the thorax, which in ancestral winged insects are ampullary enlargements of the dorsal vessel. The wing hearts of most holometabolan insects, however, are muscular diaphragms which are separate from the dorsal vessel and which are either paired or unpaired. Recently, a further vital function of the wing hearts was discovered in Drosophila, namely that they are essential for the proper maturation of wings. Toward the completion of the wing formation process, they suck epidermal cells out of the wings which are necessary before the two cuticular surfaces of each wing bond together. Flies lacking functional wing hearts never develop flight ability! Finally it should be noted that accessory hearts may also partake in the hydraulic movements
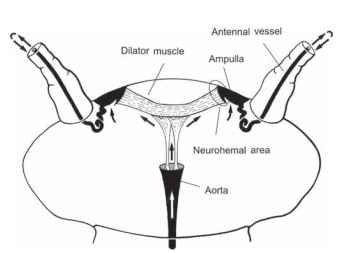
FIGURE 3 The antennal heart of the cockroach, Periplaneta americana, consists of a pulsatile ampulla at the base of each anten-nal vessel. The two ampullae expand by action of the interconnecting dilator muscle. Hemolymph rushes into each ampulla through a small ostium. When the muscle relaxes, the ampullae collapse by their own elasticity forcing the hemolymph into the antennal vessels. The hemolymph is conveyed to the end of each vessel and empties into the lumen of the antenna before returning to the head capsule; the two small muscles which extend to the anterior opening of the aorta are responsible for the suspension of the apparatus.
of body appendages. For example, the long sucking mouthparts of butterflies are uncoiled by action of special pumping organs in the head, and the lamellae of the antenna in scarabaeid beetles are spread out by action of the antennal heart.
EXTRACARDIAC PULSATIONS
First described in 1971, extracardiac pulsations of insects are the simultaneous contractions of intersegmental muscles, usually of the abdomen, that cause a sharp increase in the pressure in the insect body. The amount of movement accompanying each pulse is too small to be seen, but it can be readily measured as a slight shortening or telescoping of the abdomen as measured from its tip. The extracardiac pulses should not be confused with larger overt movements of the abdomen, especially in bees and bumble bees, that accompany ventilation during times or high activity or exertion such as flight.
Either the extracardiac pulsations occur in coordination with openings of certain of the spiracles, and therefore can play a role in ventilation, or they occur when all the spiracles are tightly closed, hence affecting hemolymph movement. The extracardiac pulsations become suspended only in quiescent stages of insect development, such as during diapause, but they can be evoked immediately upon disturbance or stimulation.
The extracardiac pulsations are driven by a part of the nervous system for which Karel Slama coined the name “coelopulse nervous system.” The pressures induced by extracardiac pulsations are 100-500 times greater than pressures caused by contractions of the dorsal vessel and are transmitted by the hemolymph throughout the entire body of the insect, influencing hemolymph movement at some distance from the dorsal vessel and APO structures.
TIDAL FLOW OF HEMOLYMPH
A special condition of the circulatory system exists in some large, high-performance flyers, such as some Lepidoptera, Diptera, and Hymenoptera. To keep body weight at a minimum the amount of
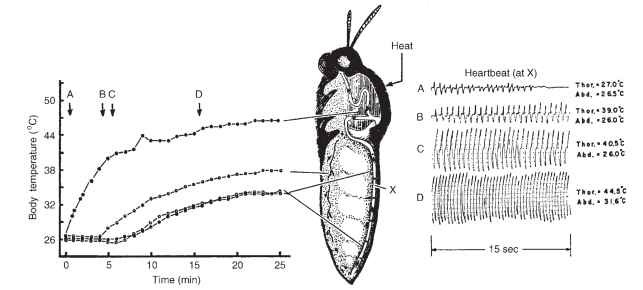
FIGURE 4 Control of thoracic temperature by central nervous control of dorsal vessel contractions during external heating of the thorax (heat). At the optimum temperature, hemolymph is pumped at maximum frequency and amplitude through the dorsal vessel to conduct heat from the thorax to the abdomen, where it is dissipated.
hemolymph is reduced and the volume replaced by large tracheal sacs. The body is often divided into two hemocoel compartments by an anatomical constriction between thorax and abdomen and may be additionally separated by a valve. The hemolymph in these insects is not circulated in the classical sense but is transported back and forth between thorax and abdomen by heartbeat reversal. The shift in hemolymph flow causes an alternating periodic increase and decrease in hemolymph volume in both compartments with a compensatory volume change in the tracheal system. This leads to ventilation especially of the large elastic tracheal sacs. Furthermore, the hemolymph in the wings oscillates in all veins simultaneously in correlation with heartbeat reversal and the resulting volume changes in the wing tracheae. Lutz Wasserthal called this periodic exchange of air and hemolymph in these insects “tidal flow” of hemolymph.
THERMOREGULATION
The use of the hemolymph in thermoregulation of flying insects was firstly described by Bernd Heinrich. The optimum temperature for flight muscle contraction in many insects, such the tobacco hornworm, Manduca sexta, is surprisingly high, up to 45°C. Before this moth can fly, it must warm the thorax to near this temperature, which it accomplishes by means of a series of simultaneous isometric contractions of the antagonistic pairs of flight muscles that appear to the casual observer as “shivering,” or vibrations of the wings (Fig. 4).
A “thermometer” in the thoracic ganglia detects the proper temperature. When the thoracic temperature is below optimum, the central nervous system signals the dorsal vessel to circulate hemol-ymph slowly. When the thoracic temperature rises above optimum, the central nervous system brings about maximal amplitude and rate of heartbeat to drive hemolymph through the thoracic muscles. The increased hemolymph flow pulls heat away from the flight muscles in the thorax and eventually delivers hot hemolymph to the abdomen, where the heat is dissipated. Then relatively cool hemolymph is redelivered to the thoracic muscles by the dorsal vessel, completing the thermoregulation cycle. The warm hemolymph is then delivered to the head and percolates back past the ventral ganglia in the thorax to the abdomen, where the heat is dissipated. The cooler hemol-ymph is then delivered again to the thorax. The dorsal vessel and the very strong ventral diaphragm in the tobacco hornworm act together to move hemolymph. When the thorax is too warm, both the amplitude and the frequency of heartbeat contractions are increased, and the rate of delivery of hemolymph increases. When the thorax is too cool, amplitude and frequency of contraction of the dorsal vessel are decreased. The activity of the ventral diaphragm acts in concert with that of the dorsal vessel.
Thermoregulation of the flight muscles of the tobacco hornworm implies a sophisticated nervous control. The overall nervous control can be easily demonstrated by severing the ventral nerve cord between the thorax and abdomen. Then the moth can no longer thermoregu-late because the feedback loop of temperature detection by the thoracic ganglia has been destroyed, and control over ventral diaphragm and dorsal vessel contractions has been lost.
AUTONOMIC NERVOUS SYSTEM
The tidal flow of hemolymph, the extracardiac pulsations, heartbeat reversal, and thermoregulation all imply a very sophisticated control of circulation by the central nervous system. The central nervous system also plays a role in regulation of the respiratory system. The activities of circulatory and respiratory systems are coordinated by the central nervous system, perhaps to an extent not fully appreciated. It would be convenient and satisfying to be able to point out a particular part of the central nervous system and related peripheral nerves in insects that might comprise this regulatory system; however, beyond evidence that the meso- and/or metathoracic ganglia play a major role in some of these functions, entomologists know of no such discrete structure or structures, possibly because these interregulatory functions have been undertaken by different parts of the nervous system in different insects. It is known that insects have a number of regulatory mechanisms that can be recruited to achieve such control, from motor and sensory neurons to neurosecretory neurons and neurohormonal organs located throughout the insect hemocoel.
