Abstract
There is increasing evidence that diabetes causes both anatomical and functional pathological changes in the myocardium. Diabetic cardiomyopathy (DC) is a myocardial disease caused by diabetes mellitus, which is unrelated to vascular pathology or systemic arterial hypertension and can occur in asymptomatic patients with diabetes alone. The coexistence of hypertension, diabetic cardiomyopathy, and myocardial ischemia increases with aging, especially among obese subjects. However relatively independent, each of these diseases seem to interact with the other in order to contribute to the biochemical, anatomical, and functional alterations in myocardial cells. The most important mechanisms involved in DC are (1) insulin resistance with consequent hyperinsulinemia and endothelial proliferation; (2) small-vessel disease (microangiopathy, impaired coronary flow reserve, and endothelial dysfunction); (3) metabolic disturbances like increased free fatty acid levels, carnitine deficiency, and changes in calcium homeostasis; (4) myocardial fibrosis (increases in angiotensin II activity, IGF-I, and inflammatory cytokines levels), and cardiac autonomic neuropathy (denervation and alterations in myocardial catecholamine levels). Abnormalities in both systolic and diastolic cardiac function have been demonstrated in diabetic subjects. Several lines of evidence indicate that left ventricular diastolic dysfunction represents the earliest preclinical manifestation of diabetic cardiomyopathy, preceding systolic dysfunction. Documentation of diastolic dysfunction should result in the initiation of therapy in order to prevent progression to Heart Failure (HF). Treatment of DC-HF is essentially not different from treating HF caused by myocardiopathies of other etiologies, and it must follow the guidelines according to ventricular function. However, some particularities related to its diabetic etiology should be considered. Degree of glycemic control correlates well with the severity of microvasculature damage, which can be delayed or even prevented by keeping near normal serum glucose levels. Achievement of ideal glycemic control levels, preferably by reducing insulin resistance, is the essential step not only in treating diabetes itself, but also in preventing and managing DC. Besides dietetic therapy, increasing levels of endurance exercise should be encouraged in order to improve peripheral insulin resistance. There is large evidence that the use of ACE inhibitors, which might reverse left ventricular hypertrophy and myocardial fibrosis, is also able to prevent myocardial remodeling, improve endothelial function, and even contribute to lower insulin resistance. It is also known that B-blockers and thiazolidinediones shift myocardium metabolism from the use of free fatty acids (FFAs) to that of glucose, which would be beneficial in DC. In addition, the thiazolidinediones have also been shown to decrease myocardial FFA levels in animals, besides improving ventricular function. In the near future, agents that decrease lipotoxicity or prevent/reverse glycosylation and cross-linking of collagen are promising.
Introduction and Epidemiology
Diabetes mellitus is the world’s fastest-growing disease with high morbidity and mortality rates, predominantly as a result of heart disease. The growing incidence, particularly of the type 2 diabetes, is alarming, especially considering the increased levels of insulin resistance and diabetes in young adults and children [1,2]. The prevalence of diabetes is growing rapidly in both the developing and the developed countries. Annually, 7 million people are newly diagnosed with diabetes mellitus in the world and more than 3.8 million deaths take place for complications associated with the disease [3,4]. Data collected by the World Health Organization (WHO) showed the prevalence of DM to be 2.8% in 2000, equivalent to 171 million persons. It projected, still, for 2030 the prevalence of 4.4 % in the worldwide population, meaning that around 366 million persons would be attacked by the disease [5]. This dramatic increase will be almost entirely due to new cases of type II diabetes [6].
The criteria for DM were sharpened, with conditions such as impaired fasting glucose (IFG) and impaired glucose tolerance (IGT) being classified as intermediate stages between the ends of the spectrum; that is, normal glucose homeostasis and diabetes. This classification is based on fasting glucose levels and glucose overload levels[7] (Table 1). Precise statistical data are lacking regarding the prevalence of IFG and IGT. Epidemiological data derived from Third National Health and Nutrition Examination Survey (NHANES III) and Diabetes Epidemiology: Collaborative analysis of diagnostic criteria in Europe (DECODE) studies, however, estimate the prevalence of these new diagnostic categories to be between 8 and 12% of the adult population [7,8].
Cardiovascular disease (CVD), including coronary heart disease (CHD), cerebrovascular disease, and peripheral vascular disease are the major causes of morbidity and the most common causes of death in people with diabetes [9]. Two-thirds of people with diabetes die of heart disease or stroke [10]. Diabetes predisposes patients to ventricular dysfunction and the development of concomitant coronary artery disease, endothelial dysfunction, hypertension, ventricular hypertrophy, coronary microvascular disease, autonomic neuropathies and metabolic abnormalities [10,11].
Heart failure (HF) is a common and serious comorbidity of diabetes. The Framingham study demonstrated the increased incidence of congestive HF in diabetic males (2.4:1) and females (5:1) independent of age, hypertension, obesity, coronary artery disease and hyperlipidaemia [12,13]. Several studies have shown that a 1% increase in HbA1c level increases the risk of developing HF by 8 to 15%, and that plasma glucose levels are associated with the risk of developing HF [7,14]. Besides, patients with diabetes account for >33% of all patients requiring hospitalization for HF [15]. Diabetic cardiomyopathy in the absence of CAD or hypertension is a common clinical feature of diabetes and is characterized mainly by diastolic dysfunction of the left ventricle and later on by a decrease of myocardial contractility [7,16].
Table 1. Definitions of normal and disturbed glucose metabolism according to World Health Organization (WHO)
|
Fasting plasma glucose (mmol/l) |
2h postload plasma |
glucose {mmol/IJ (75g glucose] |
|
 |
7.3-1 1.1 |
 |
|
 |
Normal |
IGT |
Diabetes |
 |
IFG |
IFG + IGT |
Diabetes |
 |
Diabetes |
Diabetes |
Diabetes |
IFG. impaired fasting glucose; IGT. impaired glucose tolerance.
Definitions
The condition "diabetic cardiomyopathy" was originally described in 1972 on the basis of observations in four diabetic patients who presented with heart failure without evidence of hypertension, coronary artery disease, valvular or congenital heart disease [17].
Diabetic cardiomyopathy is a clinical condition, diagnosed when ventricular dysfunction develops in patients with diabetes in the absence of coronary atherosclerosis and hypertension [18]. It refers to a disease process which affects the myocardium in diabetic patients causing a wide range of structural abnormalities eventually leading to left ventricular hypertrophy and diastolic and systolic dysfunction or a combination of these [12].
A significant number of diabetic patients exhibit diabetic cardiomyopathy. Accumulating data from experimental, pathological, epidemiological, and clinical studies have shown that diabetes mellitus results in cardiac functional and structural changes, independent of hypertension, coronary artery disease, or any other known cardiac disease, which support the existence of diabetic cardiomyopathy [18,19]. However, the frequency with which this occurs is not well defined. Although the existence of diabetic cardiomyopathy has been debated, substantial data now demonstrate that diabetes impairs ventricular function independently of other risk factors [20,21]. This specific form of cardiomyopathy has been associated with both type 1 and type 2 diabetes, but there is some evidence that it is uncommon in patients with type 1 diabetes in the era of intensive insulin therapy [22]. It is characterized by both systolic and diastolic dysfunction [4,23], presenting clinically with impaired diastolic function developing first [2,24].
Evidence now indicates that this cardiomyopathy is also seen in patients and animals predisposed to diabetes but presenting only with metabolic complications associated with insulin resistance [25]. Studies have suggested that increased risk of cardiovascular disease is not restricted to type II or type I diabetes mellitus, but extends to pre-diabetic stages such as impaired fasting glucose, impaired glucose tolerance, metabolic syndrome, and obesity. Insulin resistance impaired fasting glucose, impaired glucose tolerance, and diabetes mellitus would form a continuous sequence of risk for cardiovascular disease [7].
During diabetes, changes in cardiac metabolism occur early and precede the development of cardiomyopathy. Even though altered metabolism is inadequate to produce cardiac functional changes at this early time, it is likely that early metabolic damage is occurring at the cellular or subcellular levels. Overtime, these cumulative defects could be contributing to diabetic cardiomyopathy [26].
Pathophysiology
The three characteristic metabolic disturbances evident in diabetic states are hyperlipidemia (usually in the form of increased triglycerides and free fatty acids [FFAs]), early hyperinsulinemia followed by pancreatic-cell failure, which leads eventually to hyperglycemia [18]. The increase of triglycerides, insulin and glucose induces alterations in the activation of cellular transcription in the myocardium, altering the use of substrates, myocardium growth, and leading to endothelial dysfunction and increases stiffness [27]. Alterations in body mass (obesity) and adipocytokines (leptin, adiponectin) have also been implicated in the cardiovascular pathophysiology observed in diabetes. As such, the effects of increased FFAs, altered insulin action, and hyperglycemia can be considered triggers to the cardiac phenotype in diabetes [18].
Alterations in Substrate Supply and Utilization
Metabolic changes in diabetes are directly triggered by hyperglycemia. Increasing evidence suggests that altered substrate supply and utilization by cardiac myocytes could be the primary injury in the pathogenesis of this specific heart muscle disease [19,28]. A significant reduction in myocardial glucose supply and utilization has been observed in isolated diabetic cardiomyocytes [19] and diabetic patients [19,29]. A major restriction to glucose utilization in the diabetic heart is the slow rate of glucose transport across the sarcolemmal membrane into the myocardium, probably due to the cellular depletion of glucose transporters (GLUTs) 1 and 4, which can be corrected by insulin therapy [19,30]. A second mechanism of reduced glucose oxidation is via the inhibitory effect of fatty acid oxidation on pyruvate dehydrogenase complex due to high circulating FFAs. This has the net effect of reducing ATP availability and may be more important in type II diabetes, in which FFAs levels tend to be higher. The potential importance of this mechanism is exemplified by the observation that diabetic animals with minimal hypertriglyceridemia are resistant to the development of cardiomyopathy [31,32]. Both of these pathological mechanisms are potentially reversible in a short time frame, and the dynamics of each mechanism is compatible with the observation that cardiac dysfunction may be improved with improved metabolic control [19].
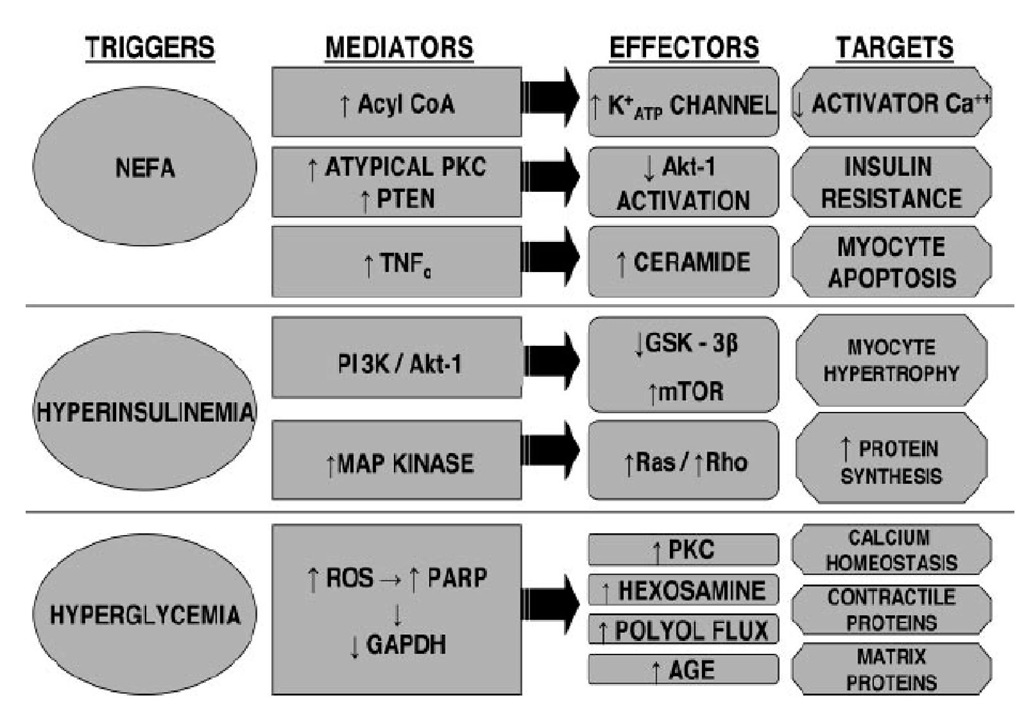
Figure 1. DIABETES LEADS TO CARDIOMYOPATHY. GAPDH: glyceraldehyde phosphate dehydrogenase; GSK-3B: glycogen synthases kinase-3B; MAP: mitogen-activated protein; PARP: poly (ADP ribose) polymerase; PKC: protein kinase C; PI3K: phosphatidylinositol 3-kinase; PTEN: phosphatase and tensin homolog; ROS: reactive oxygen species; TNF: tumor necrosis factor.
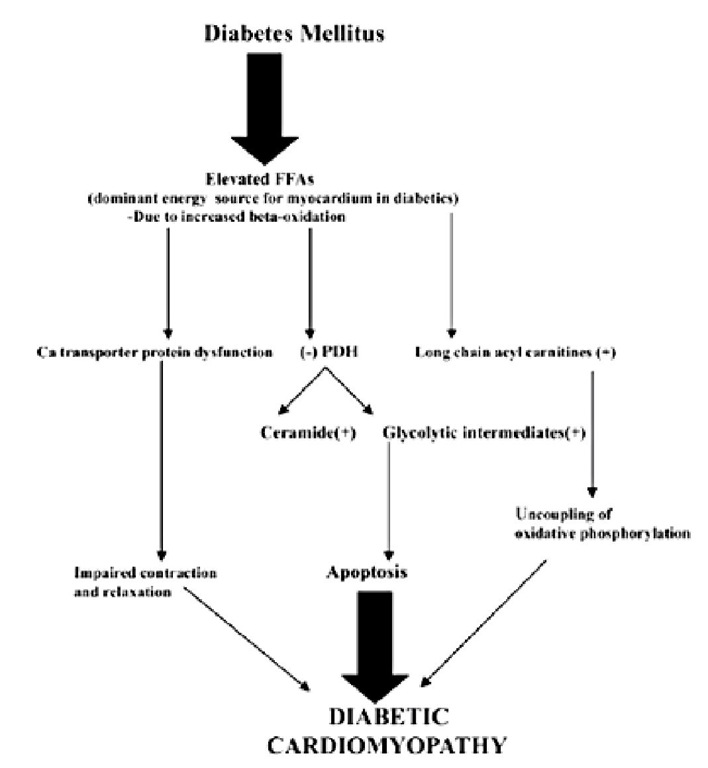
FFA metabolism: Elevated FFA levels are believed to be one of the major contributing factors in the pathogenesis of diabetes. FFAs enhance peripheral insulin resistance and trigger cell death. Disturbances of FFA metabolism may be an important contributor to abnormal myocardial function in diabetes. These changes are characterized by elevation of circulating FFAs caused by enhanced adipose tissue lipolysis, as well as high tissue FFAs caused by hydrolysis of augmented myocardial triglyceride stores. Moreover, in addition to the FFA-induced inhibition of glucose oxidation, high circulating and cellular FFA levels may result in abnormally high oxygen requirements during FFA metabolism and the intracellular accumulation of potentially toxic intermediates of FFAs, all of which lead to impaired myocardial performance and severe morphological changes [19]. FFAs play a central role in altering cellular insulin signaling through several mechanisms leading to insulin resistance and compensatory hyperinsulinemia [18,33,34]. Abnormalities in FFA metabolism have been demonstrated in idiopathic dilated cardiomyopathy in which the rate of FFA uptake by myocardium is inversely proportional to the severity of the myocardial dysfunction [35]. It is possible that similar defects contribute to the development of diabetic cardiomyopathy. The FFA-induced impairment of glucose oxidation may be a major factor in the development of diabetic cardiomyopathy, and would explain why cardiac function tends to improve upon metabolic improvement. Furthermore, the availability of carnitine, an essential substance for myocardial FFA metabolism, is usually reduced in diabetes [19]. Conversely, normalizing cardiac metabolism in diabetic animals reverses the development of cardiomyopathy [36,37].
Figure 2. The role of altered myocardial metabolism in the development of diabetic cardiomyopathy. FFA = free fatty acid; PDH = pyruvate dehydrogenase.
Lipotoxicity: Lipotoxicity is the process by which excess fatty acids and associated triglyceride accumulation in parenchymal cardiac myocytes cause cellular dysfunction and death, and eventual myocardial dysfunction. An imbalance between fatty acid uptake and use leads to the inappropriate accumulation of free fatty acids and neutral lipids within cardiomyocytes. Long-chain nonesterified fatty acids and their products, such as ceramides and diacylglycerols, cause the majority of the toxic effects [38,39]. A number of studies have suggested that excessive fatty acid overload induces lipotoxicity and contributes to the initiation and development of cardiomyopathy [26,37,40]. With the use of transgenic mice, studies have shown that elevation of fatty acid uptake or utilization induces lipotoxicity in the absence of any systemic metabolic disturbance [26].
The mechanisms that mediate cardiac lipotoxicity are still not completely understood. One potential target is over production of reactive oxygen species (ROS) [41]. High rate of fatty acid oxidation increases mitochondrial action potential, leading to augmented ROS generation. Another potential mechanism for lipotoxicity is accumulation of lipids, when fatty acid uptake supersedes its oxidation. Regarding accumulation of triglycerides, the role of this neutral lipid in inducing contractile dysfunction is still unknown, although a strong association between triglycerides storage and lipotoxicity has been established in both animal models and human studies [42,43]. Taken together, there is strong evidence for lipotoxic mechanisms in rodents showing that lipid accumulation in the heart leads to heart failure [44]. Data indicate that the cardiac accumulation of triglycerides is related to FFA exposure, generalized ectopic fat excess, and peripheral vascular resistance and that these changes precede left ventricle overload and hypertrophy [44,45].