Echocardiography
Echocardiography associated with color flow Doppler is the most effective test for the diagnosis of hypertrophic cardiomyopathy (B.J. Maron et al., 2003a). It allows detection of the disease, follow-up of progression, and risk stratification for sudden death (B.J. Maron et al., 1986, Ostman-Smith et al., 2005). The wall thickness echocardiographic criteria for the diagnosis of hypertrophic cardiomyopathy was set at greater than 2 SD above the mean for the body surface area of the population for a localized or general myocardial hypertrophy (Grenier et al., 2000). When in the absence of pulmonary valve stenosis the right ventricular wall thickness exceeds 4 mm the right ventricle is considered to be involved too (Nugent et al., 2005). Left ventricular hypertrophy is most frequently asymmetric with greater involvement of the interventricular septum than the rest of the walls, though it can also be concentric. (Fig. 9 – 11) More rarely, it is localized in the anterior wall, the apex, or in the mid left ventricle (M. Maron et al., 2009, Minami et al., 2011). The mid ventricular obstruction may lead to the development of an apical left ventricular aneurysm. (Fig. 12) In younger children the right ventricle may also be affected (Biagini et al., 2005). As a consequence of mitral insufficiency and/or diastolic dysfunction, there is left atrial enlargement. The systolic anterior movement of the mitral valve contacting the ventricular septum that causes left ventricular outflow tract obstruction in patients with hypertrophic obstructive cardiomyopathy is readily seen (B.J. Maron et al., 2003). Color flow mapping allows detection of the site of obstruction. (Fig. 13) The gradient across the outflow tract is estimated by continuous wave Doppler that also allows assessment of the mitral regurgitation severity. (Fig. 14) Transesophageal echocardiography is more sensitive than transthoracic studies for evaluation of primary mitral valve anomalies producing mitral incompetence (Kuhl & Hanrath, 2004). The presence of left ventricular outflow tract obstruction is now considered a risk factor for adverse events in hypertrophic cardiomyopathy (M. Maron et al., 2003). Furthermore, we now know that almost 70% of patients with hypertrophic cardiomyopathy have gradients across the left ventricular outflow considering the obstruction caused by exercise, when studied with stress echo. Actually, it should be performed in all patients with no significant gradient at rest (M. Maron et al., 2006). Estimation of diastolic dysfunction in hypertrophic cardiomyopathy is performed by studying the pulmonary vein and transmitral Doppler flow tracings but since they depend on loading conditions are not reliable to predict adverse outcomes in children with hypertrophic cardiomyopathy (McMahon et al., 2004). Tissue Doppler velocities measurements at the mitral annulus level are more sensitive in detecting diastolic dysfunction allowing early diagnosis in hypertrophic cardiomyopathy genetic carriers before they develop hypertrophy (Nagheb et al., 2003). (Fig. 15) Tissue Doppler studies can also predict adverse events like death, ventricular tachycardia, cardiac arrest, and exercise intolerance in affected children with the disease (McMahon et al., 2004).
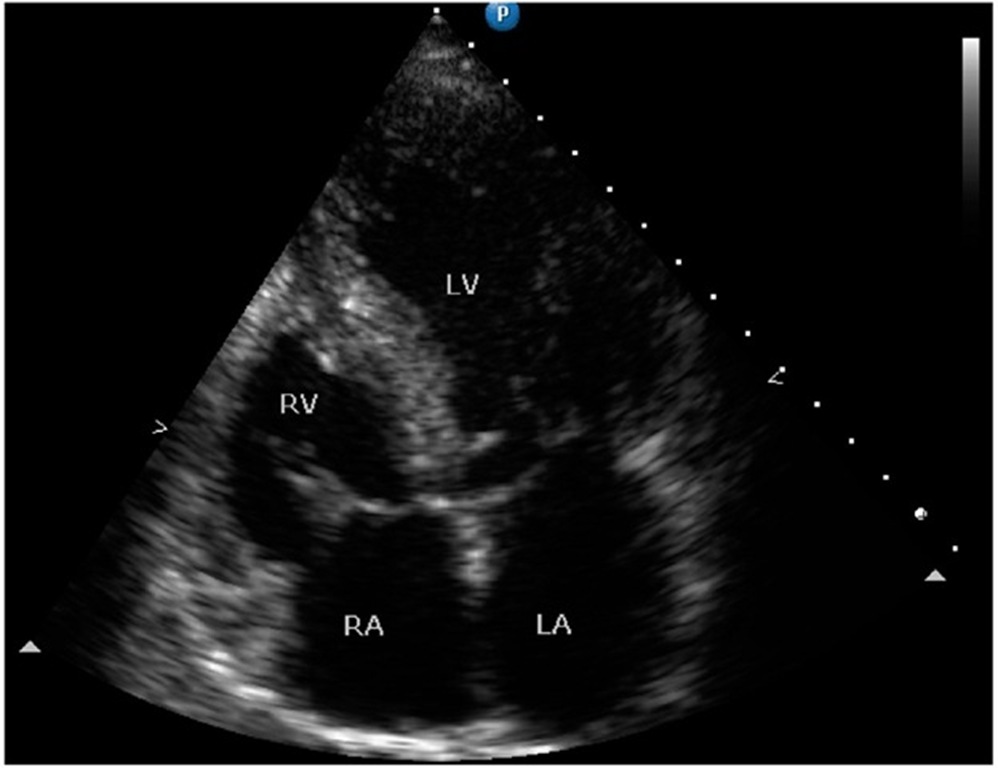
Fig. 9. Four-chamber bidimensional echocardiographic view of a 16 year-old asymptomatic male patient with hypertrophic cardiomyopathy with asymmetric septal hypertrophy. The septum and the posterior wall measure 21 mm and 9.5 mm respectively.
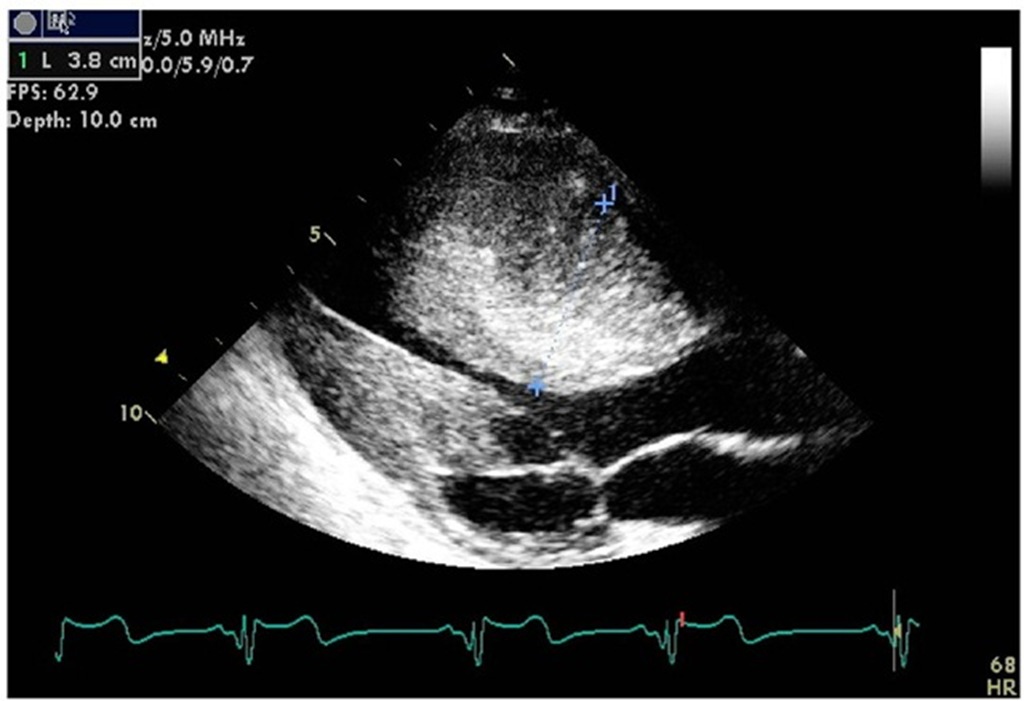
Fig. 10. Bidimensional echocardiogram showing long (A) and short (B) axis parasternal views of the left ventricle of a 22 year-old female followed since early childhood with severe asymmetrical hypertrophy of the septum measuring 31 mm in diameter. There is convexity toward the left ventricular cavity. The mitral leaflets initiate an anterior motion to provoke mitral septal contact and the resultant left ventricular outflow tract obstruction. The left atrium is mildly enlarged. The tip of a catheter for DDD pacing is seen in the right ventricular cavity (arrows).
Fig. 11. Bidimensional echocardiographic long axis view with massive septal hypertrophy (38 mm) in a girl with a strong family history (2 siblings).
Fig. 12. Bidimensional echocardiographic view of a patient with midventricular obstruction. The left ventricle has an upper inflow (*) and a lower apical chamber (#) as a result of the obstruction.
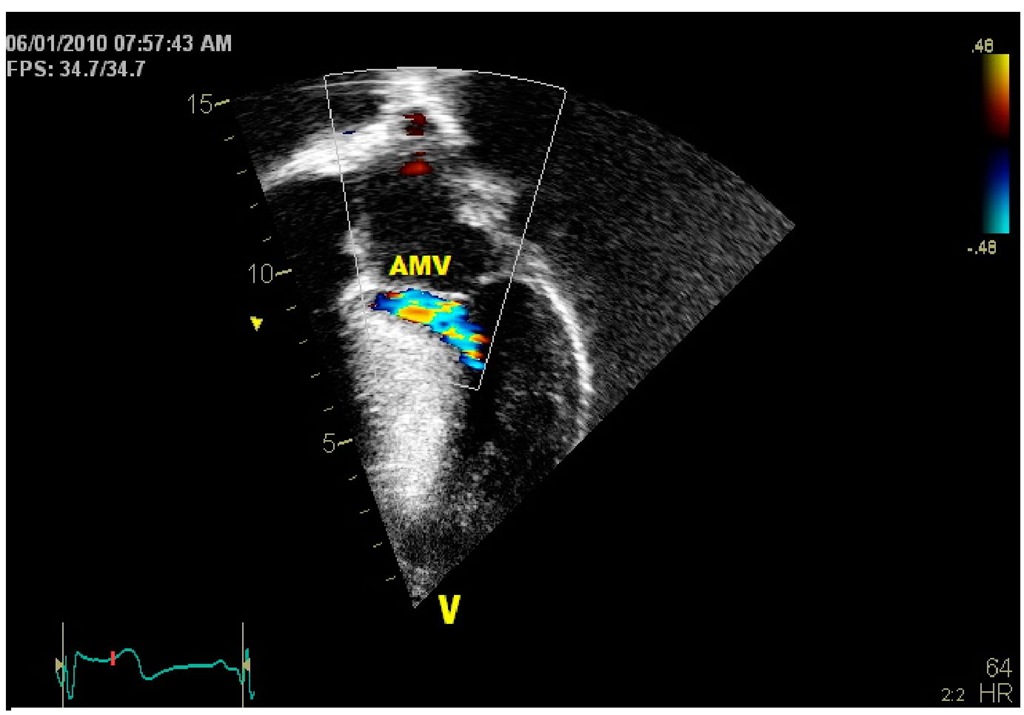
Fig. 13. Echocardiographic 4-chamber view of asymmetric septal hypertrophy with left ventricular outflow tract flow acceleration (arrow) by color Doppler. (AMV: anterior mitral valve).
Fig. 14. Continuous wave Doppler showing a severe gradient (87.6 mmHg) across the left ventricular outflow tract in the same patient shown on figure 10.
Fig. 15. Decreased Doppler tissue septal velocities (<5cm/ second) in a patient with massive hypertrophic cardiomyopathy.