Clinical findings
Aside from the genetic and phenotypic heterogeneity already mentioned in hypertrophic cardiomyopathy, the age, form of presentation, and outcome, are also quite diverse. The age of the patient at presentation is a determinant of prognosis (Colan et al., 2007). Newborn and infants are more likely to be referred for congestive heart failure while older children are usually asymptomatic at the time of diagnosis and the consultation is requested because of the presence of a heart murmur, cardiomegaly casually detected on a chest x-ray, or electrocardiographic abnormalities (Bruno et al., 2002). Initially, asymptomatic patients may go on without symptoms for a long period of time until they begin experiencing fatigue and dyspnea, and less frequently, palpitations, chest pain, and syncope or sudden death which might be the first symptom ever. Another cause of referral is the investigation of cardiac involvement in patients with conditions known to be associated with hypertrophic cardiomyopathy like the malformation syndromes, inborn errors of metabolism or neuromuscular disorders. However, in other circumstances, the associated disease may have been unnoticed and the cardiomyopathy is discovered first. The presence of physical findings suggesting an association then prompts referral to the geneticist (Alday & Moreyra, 1984). With regard to the physical examination itself, in hypertrophic obstructive cardiomyopathy, the peripheral pulses may rise and descend rapidly. When considerable cardiac enlargement is present, a precordial bulge and outward displacement of the point of maximal impulse are usually found. A double apical impulse caused by a 4th heart sound is often noted. A harsh, blowing systolic murmur with varying intensity, related to the degree of obstruction and mitral regurgitation, can be listened along the left sternal border and at the apex. A distinctive feature of these murmurs is the variability seen with maneuvers that increase or reduce the obstruction intensifying or decreasing their intensity (Moreyra et al, 1972). (Fig. 4) Patients with non obstructive hypertrophic cardiomyopathy may also have ejection systolic murmurs along the left sternal border though softer than when there is left ventricular outflow obstruction. An ambulatory electrocardiographic study performed in infants, children and adolescents with hypertrophic cardiomyopathy concluded that arrhythmias occur rarely before adolescence. However, from then on, prevalence of nonsustained ventricular tachycardia is as high as 18%. It is also worth mentioning that absence of arrhythmias is not synonymous of low risk for sudden death (McKenna et al., 1988). Supraventricular and ventricular tachycardias may be equally found. Atrial fibrillation may occur in end-stage dilated hypertrophic cardiomyopathy (Harris et al., 2006). Very rarely, patients with hypertrophic cardiomyopathy have associated Wolff-Parkinson-White syndrome (Bockowski et al., 2007). The development of high rate supraventricular tachycardias is poorly tolerated in these patients with left ventricular diastolic dysfunction. This might lead to syncope and sudden death which are the most feared complications of hypertrophic cardiomyopathy. Several mechanisms have been reported as a cause of syncope like tachyarrhythmias, left ventricular outflow tract obstruction, systemic hypotension, and 3rd degree AV block. Sudden unexpected death may be the first and only symptom in hypertrophic cardiomyopathy and this is the most frequent cause of death in young athletes during competition. However, sudden death also occurs during usual activities, at rest, or during sleep time. The estimated rate of sudden death in children is 3% per year, a similar figure to that found in adults cared for in tertiary centers (Bruno & al., 2002; B.J. Maron et al., 1982; McKenna & Deanfield, 1984). Heart failure in this disease is usually provoked by diastolic dysfunction secondary to left ventricular hypertrophy, myocyte disarray and fibrosis. The presence of outflow obstruction is an additional factor leading to heart failure. In 3 to 5 % of patients the end stage is reached and systolic failure associated with left ventricular wall thinning and increased ventricular volume become a serious indication of poor prognosis (Biagini et al., 2005 & Harris et al., 2006).
Fig. 4. Simultaneous hemodynamic recordings of the left ventricular inflow (LV in), aorta (Ao) and main pulmonary artery (MPA). An electrocardiogram (ECG) and a phonocardiogram (phono) were also recorded. There is a small basal gradient between the left ventricular inflow and the aorta which increases in a post-extrasystolic beat together with the intensity of the systolic murmur shown in the phonocardiogram, as a consequence of the stronger contraction following the post-extrasystolic pause. (IHSS: idiopathic hypertrophic subaortic stenosis).
Laboratory studies
Radiology
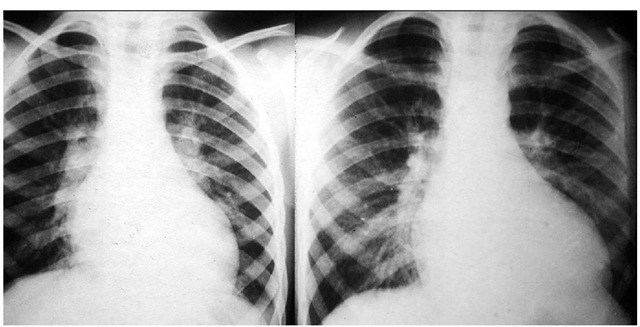
The chest x-ray is usually normal in early stages of the disease but following the pubertal growth spurt shows cardiomegaly at the expense of the left sided chambers (B.J. Maron et al., 1986). (Fig. 5) The same is true for patients evolving to end-stage hypertrophic cardiomyopathy (Biagini et al., 2005). Finally, infants with severe heart failure nearly always have considerable cardiac enlargement at presentation. Pulmonary venous hypertension secondary to elevation of the left ventricular end diastolic pressure is reflected by the well known resultant pulmonary vascular changes.
Electrocardiography and allied techniques
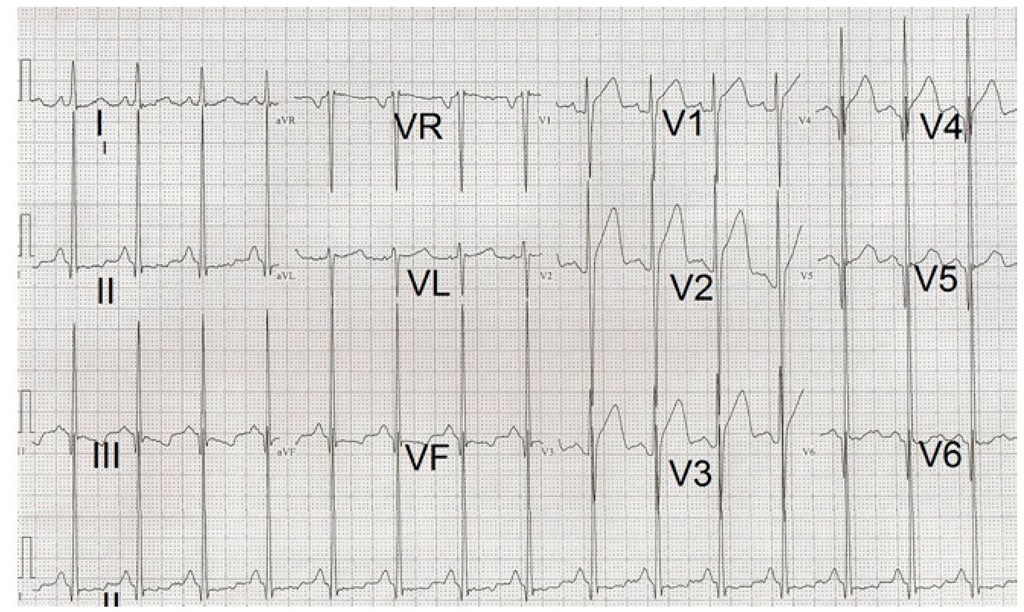
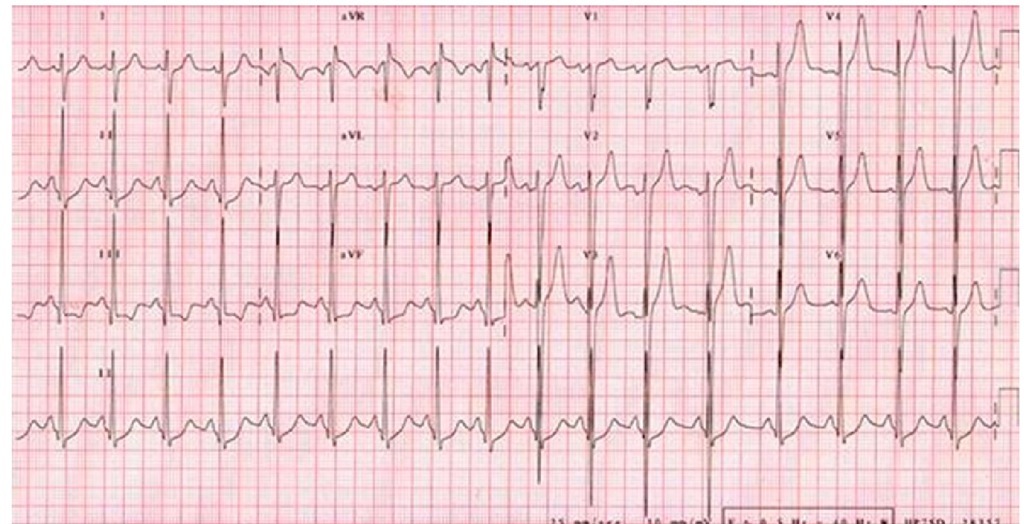
The electrocardiogram is usually abnormal in almost all patients with hypertrophic cardiomyopathy. (Panza & Maron, 1989) The electrocardiographic abnormality, even a slight one, may precede the echocardiographic findings showing left ventricular hypertrophy in family members carrying the genotype of probands with hypertrophic cardiomyopathy. Therefore, this should be kept on mind when screening relatives of hypertrophic cardiomyopathy patients (Gregor et al., 1989). Left atrial enlargement and left ventricular hypertrophy by voltage criteria usually associated with ST-T abnormalities are seen. (Fig. 6) Younger patients may have combined ventricular or isolated right ventricular hypertrophy with a rightwards QRS axis. (Fig. 7) Another frequent finding is the presence of pathological deep and narrow Q waves. An R + S sum higher than 10 mV on the limb leads of the electrocardiogram has been recently proposed as a risk factor for sudden death in children with hypertrophic cardiomyopathy (Ostman-Smith et al., 2005). (Fig. 8) An electrocardiographic Wolff-Parkinson-White pattern or syndrome is very rare in patients with sarcomeric hypertrophic cardiomyopathy. However, it has been described in mutations of genes like those encoding AMP-activated protein kinase (PRKAG2) and lysosome associated membrane protein 2 (LAMP2) producing glycogen storage disease and Danon disease respectively. Both of them recognized as phenocopies of hypertrophic cardiomyopathy (Alday et al., 2010). Ambulatory electrocardiography and exercise testing are used for arrhythmia detection and risk stratification in older children with hypertrophic cardiomyopathy. The presence of nonsustained ventricular tachycardia by Holter monitoring or an abnormal blood pressure response to exercise are considered risk factors for sudden death (Elliot et al., 2000).
Fig. 5. Chest x-rays of a boy at 11 and 13 years of age showing great increase in the heart size coincident with pubertal growth spurt.
Fig. 6. Electrocardiogram of a 16 year-old male patient showing increased R wave voltages and secondary ST-T changes indicating severe left ventricular hypertrophy.
Fig. 7. Electrocardiogram belonging to a 4 year-old boy with a QRS axis of -120° with Q waves in leads II, III and aVF. There are signs of combined ventricular hypertrophy, loss of R wave voltage from V4 to V8 with appearance of QS complexes in V6 and pathological Q waves in V7 and V8.
Fig. 8. Electrocardiogram of a 14 year-old male patient with a vertical QRS axis (+120°) and voltage criteria for left ventricular hypertrophy. A limb lead voltage sum > 10 mV is considered a risk factor for sudden death in children.