Introduction
The definition and classification of the cardiomyopathies has been traditionally a complex and quite variable subject. In 2006, the American Heart Association issued a scientific statement elaborated by a task force of experts that contemplated the important development of molecular genetics in recent years, to explain the etiology of the diseases of cardiac muscle, or cardiomyopathies, previously considered idiopathic (B.J. Maron et al., 2006a). The document stated that "Cardiomyopathies are an heterogeneous group of myocardial diseases associated with mechanical and/or electrical dysfunction that usually, but not always, exhibit inappropriate ventricular hypertrophy or dilation, and are originated by a variety of causes, frequently genetic. Cardiomyopathies involve just the heart or are part of systemic disorders that often lead to cardiovascular death or heart failure related disability". Myocardial damage secondary to coronary atherosclerosis, heart valve disease, congenital heart disease, and systemic hypertension, is excluded from this definition. Primary or metastatic cardiac tumors and diseases primarily affecting the endocardium with minimal or absent myocardial damage neither are included. The document also discourages the use of the classical terminologies hypertrophic, dilated, and restrictive cardiomyopathies because they have overlapping features and often mutate from one type to another during the course of the disease. Cardiomyopathies are then classified into 2 groups, primary when there is only heart involvement, and secondary if the heart is affected by systemic diseases with multiorganic involvement. (Table 1) Primary cardiomyopathies are divided into genetic, acquired, and mixed (genetic and acquired).
|
GENETIC |
MIXED |
ACQUIRED |
|
Hypertrophic |
Dilated |
Inflammatory |
|
Arrhythmogenic Right Ventricular |
Restrictive |
Tako-tsubo |
|
Noncompaction |
Peripartum |
|
|
Glycogen Storage |
Tachycardia-induced |
|
|
Mitochondrial |
Infants of diabetic mothers |
|
|
Conduction Defects |
||
|
Ion Channelopathies |
Table 1. Classification of the primary cardiomyopathies
A salient feature of this classification is the inclusion of ion channelopathies caused by gene coding mutations of Na, K, and Ca channels. These channelopathies may result in deadly ventricular arrhythmias and can only be identified by molecular genetic studies since no structural cardiac damage is objectified. The Brugada syndrome, the long and short QT syndromes, the cathecolaminergic polymorphic ventricular tachycardia, and the unexplained nocturnal sudden death in Southeastern Asian youngsters belong to the channelopathies. Some conduction disorders are also included in the classification. In contrast with the American Heart Association point of view, the European Society of Cardiology issued a report in the year 2008 with an updated definition and classification of the cardiomyopathies (Elliot et al., 2008). (Table 2) It was there stated that "Cardiomyopathies are structural and functional myocardial diseases in the absence of systemic hypertension, coronary atherosclerosis, valvulopathies, or congenital heart disease sufficient to explain the observed abnormality". Therefore, hypertrophic cardiomyopathy was defined as "Increased ventricular thickness or mass in the absence of loading conditions sufficient to cause the observed abnormality". This definition better reflects the terminology used in pediatrics (Elliot et al., 2008; Franklin et al., 1999). With regard to the classification, it was based on the identification of phenotypes according to their structural and functional features recognizing the following cardiomyopathies: hypertrophic, dilated, restrictive, arrhythmogenic right ventricular, and unclassified (Colan et al., 2007). Every phenotype could be familial or non-familial emphasizing the role of genetics in some cardiomyopathies and orienting the etiologic diagnosis. The differentiation between primary and secondary cardiomyopathy is then abandoned. Left ventricular non-compaction and the takotsubo cardiomyopathy are included in the group of unclassified cardiomyopathies. The European Society of Cardiology experts do not believe that channelopathies and conduction disorders should be considered as cardiomyopathies. In our opinion, the European Society of Cardiology classification is more user-friendly for general physicians.
HCM: hypertrophic cardiomyopathy, DCM: dilated cardiomyopathy, ARVC: arrhythmogenic right ventricular cardiomyopathy, RCM: restrictive cardiomyopathy.
Table 2. European Society of Cardiology classification of primary cardiomyopathies.
Classification
Though hypertrophic cardiomyopathy was first recognized by Liouville in France in 1869, (Liouville, 1869, as cited in Marian, 2007), it was not until the 1950′s that was rediscovered in Britain by Brock and Teare (Brock & Fleming, 1956; Teare, 1958). Initial reports emphasized the presence of left ventricular outflow tract obstruction until it was realized that this could be absent (B. J. Maron et al, 2009). Since then, two main types of hypertrophic cardiomyopathy were distinguished, with or without obstruction. Nowadays, we know that hypertrophic cardiomyopathy, is the most frequent monogenic disorder in cardiology and the commonest cause of sudden death in youngsters in either form of presentation (J. Seidman & C. Seidman, 2001).
Regardless of the presence or absence of obstruction, hypertrophic cardiomyopathy is classified into two main groups, familial and non-familial. (Table 3) The latter comprises 4 subgroups: hypertrophic cardiomyopathy associated with obesity, infants born to diabetic mothers, athlete’s heart, and amyloidosis. This topic will mainly address the familial forms of hypertrophic cardiomyopathy also composed of 4 subgroups: sarcomeric, and 3 others in association with malformation syndromes, inborn errors of metabolism, and neuromuscular disorders (Elliot et al., 2008). The sarcomeric forms are the most frequent and have an autosomal dominant inheritance. They are caused by missence mutations of genes encoding the contractile proteins of the sarcomere. A mutation involves the change of a DNA base for another resulting in the replacement of an aminoacid in a polypeptide for another. Though readable, the meaning (sense) of the genetic message is changed. Considerable genetic and phenotypic heterogeneity is found in hypertrophic cardiomyopathy. In other words, different gene mutations may cause similar phenotypes or on the contrary, the same gene may result in dissimilar ones. The presence of modifying genes, like that encoding angiotensin II, environmental influences, gender, and associated conditions might explain some of these dissimilarities (Alcalai et al., 2008). About 20 genes carrying a great number of mutations have already been identified in hypertrophic cardiomyopathy (Kim et al., 2011). (Table 4) However, just 3 of them, beta-myosin heavy chain (MYH7), myosin binding protein C (MYBPC3), and troponin T (TNNT2) are responsible for almost 75% of the cases, thence, the remaining are rare. The genes involved in pediatric hypertrophic cardiomyopathy have a similar frequency and distribution as in adult patients (Kaski et al., 2009).
|
FAMILIAL |
NON-FAMILIAL. |
|
Sarcomeric |
Associated with obesity |
|
Associated with malformation syndromes |
Infants born to diabetic mothers |
|
Associated with inborn errors of metabolism |
Athlete’s heart |
|
Associated with neuromuscular disorders |
Amyloidosis |
Table 3. Classification of the hypertrophic cardiomyopathies.
It has been suggested that the genotype might have an influence in the prognosis in hypertrophic cardiomyopathy. Patients with MYH7 mutation would have more severe ventricular hypertrophy and present earlier in life, those with TNNT2 would have less left ventricular hypertrophy but higher risk of sudden death, and late onset of the disease and favorable prognosis would be found in patients with MYBPC3 mutation (Moolman et al., 1997; Niimura et al., 1998; Watkins et al., 1992). Nevertheless, a more recent study showed that regardless of the gene mutation, patients with a positive molecular genetic study, had a higher risk of cardiovascular death, stroke, worse functional class, diastolic and systolic left ventricular dysfunction, and that the long term outcome was worse for patients carrying more than one mutation. (Bos et al., 2009) The latter finding was not corroborated in children. (Kaski et al., 2009).
|
GENE |
PROTEINS |
GENE |
PROTEINS |
|
MYH7 |
P-Myosin heavy chain |
TTN |
Titin |
|
MYH6 |
a-Myosin heavy chain |
LBD3 |
LIM binding domain 3 |
|
MYBPC3 |
Cardiac myosin binding protein C |
CSRP3 |
Muscle LIM protein |
|
TNNT2 |
Cardiac troponin T |
TCAP |
Telethonin |
|
TNNI3 |
Cardiac troponin I |
VCL |
Vinculin/metavinculin |
|
TNNC1 |
Cardiac troponin C |
ACTN2 |
a-Actinin 2 |
|
TPM1 |
a-Tropomyosin |
MYOZ2 |
Myozenin 2 |
|
MYL3 |
Myosin essential light chain |
JPH2 |
Junctophillin-2 |
|
MYL2 |
Myosin regulatory light chain |
PLN |
Phospholamban |
|
ACTC |
a-Cardiac actin |
Table 4. Susceptibility genes in hypertrophic cardiomyopathy.
Prevalence
The estimated prevalence of hypertrophic cardiomyopathy in the adult population as assessed by echocardiography screening is 1:500 (B.J. Maron et al., 1995a). However, in pediatrics, the observed prevalence is much lower because hypertrophic cardiomyopathy usually has late gene expression. Large population registries from Australia and the US show a prevalence varying between 0.47 and 1.24:100,000 inhabitants and an occurrence of nearly 25% among all types of cardiomyopathies (Lipschultz et al., 2003; Nugent et al., 2003). In our institution, the incidence of hypertrophic cardiomyopathy was 1.1% for all children with heart disease attending the Division of Cardiology of the Children’s Hospital (Bruno et al., 2002).
Pathology
Macroscopic findings
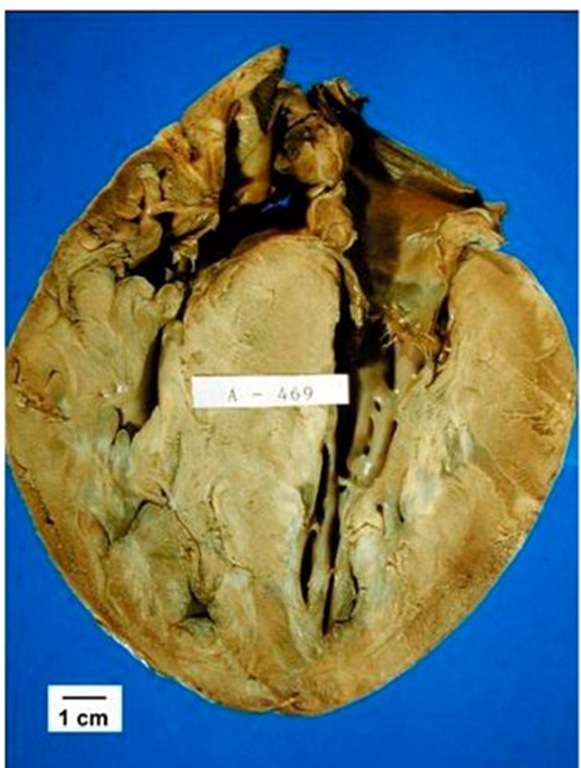
The gross anatomy generally shows severe left ventricular hypertrophy and small cavity size. (Fig. 1) The hypertrophy mainly involves the ventricular septum, and for this reason, one of the early denominations of the disease was asymmetric septal hypertrophy (Henry et al., 1973). Notwithstanding, hypertrophy may occur symmetrically or affect other segments like the posterior wall, and the apical or middle sections of the left ventricle (Falicov & Resnekov, 1977; Louie & Maron, 1987; Minami et al., 2011; Yamaguchi et al., 1979). Midventricular obstructive hypertrophic cardiomyopathy is more frequent in Asians with a prevalence of around 10% in tertiary centers and carries a higher risk for adverse events (B.J. Maron et al., 2003a; Minami et al., 2011). Patients with apical involvement are less commonly genotype positive than those with the more frequent variants of the disease but the affected genes are usually the same frequently found in the other patients (MYBPC3 and MYH7) (Gruner et al., 2011). In infants and children, the right ventricle can also be involved (Biagini et al., 2005).Almost 5% of patients with hypertrophic cardiomyopathy evolve to end stage dilated cardiomyopathy with extensive fibrosis, myocardial wall thinning and cavity dilation (Harris et al., 2006).
Fig. 1. Longitudinal section of the heart of a 9 year-old boy, who died suddenly during ordinary activities, with predominant hypertrophy of the septum but also showing increased thickness of the free wall of both ventricles. During life, obstruction of both the left and right ventricular outflow tracts was present.
The left atrium is enlarged as a consequence of the elevated left ventricular end diastolic pressure caused by diastolic dysfunction and mitral regurgitation secondary to left ventricular outflow tract obstruction or associated mitral valve anomalies (Klues et al., 1992). The physiopathology of mitral insufficiency in hypertrophic obstructive cardiomyopathy was initially attributed to the Venturi effect produced by systolic flow acceleration in the left ventricular outflow tract dragging the anterior mitral valve leaflet towards the ventricular septum causing both obstruction and insufficiency (Grigg et al., 1992; Panza et al., 1992; Shah et al., 1969 & 1971). A subsequent echocardiographic and Doppler study suggested instead, that the mitral valve leaflets are protruding into a narrow left ventricular outflow tract at the onset of ejection causing that rapid forward flow becomes the dominant force that pushes the leaflets toward the septum being the immediate cause of obstruction. After the onset of obstruction the leaflets are forced against the septum by the pressure difference across the orifice. The raising gradient leads to a smaller orifice and a higher gradient (Sherrid et al., 1993). The systolic anterior motion of the mitral leaflets precludes the proper sealing of the mitral orifice generating mild to moderate mitral regurgitation (M. Maron et al., 2011). The mitral valve in these patients shows alterations in size and shape which are thought to be primary abnormalities of the disease. The main changes are elongation and increase of the leaflet area usually not symmetrical. The size of the left ventricular outflow, the hyperdynamic contraction and the alterations of the mitral valve are the causes of the obstruction.
Microscopy
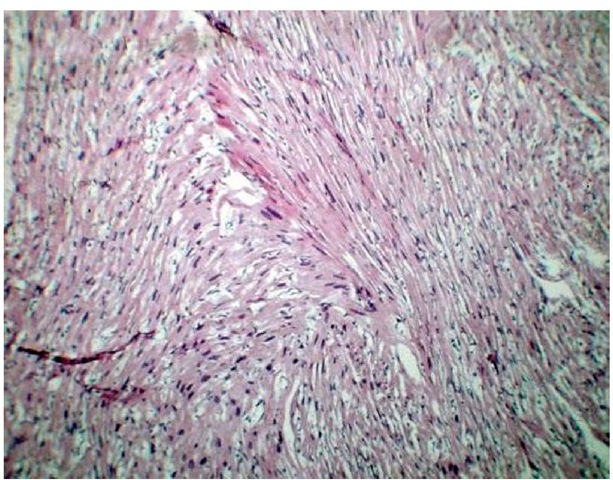
The distinct feature of the microscopic examination of the myocardium is hypertrophy and marked disarray (greater than 5% of the myocardial tissue) of individual and grouped myocardiocytes (myofibers) that instead of being normally aligned are interspersed in different directions forming whorls around areas of fibrosis. Cells and fibers lose their normal parallelism and can even be found almost perpendicular to each other. (Fig. 2) The disarray also includes the intracellular myofibrils. Other findings include increased connective tissue leading to interstitial fibrosis and thickening of the microvascular coronary artery walls with luminal reduction resulting in ischemia and fibrosis (Ferrans et al., 1972). Fibrosis and scar replacement of necrosed cells is more evident in areas with greater hypertrophy. Initially, it was postulated that the mechanism for the disarray and hypertrophy was caused by the increased effort of the myocytes to compensate the inefficient contractility of the affected sarcomere proteins. This would activate the insulin and tissue growth factors and angiotensin II resulting in the myocardial changes (J. Seidman & C. Seidman, 2001). Further experimental animal investigations and studies of hypertrophic cardiomyopathy mutations in man, by the same authors, found instead that the mutated sarcomeres had in fact increased function. It was then hypothesized that they would activate signals for hypertrophic remodeling. Abnormalities in calcium signaling were encountered leading to necrosis and replacement fibrosis producing diastolic dysfunction, a main feature of hypertrophic cardiomyopathy (C. Seidman & J. Seidman, 2011). An investigation by the same group, also found that a profibrotic marker like serum procollagen is significantly higher in patients with full blown hypertrophic cardiomyopathy and mutation carriers, with a still not developed phenotype, than in controls, pointing to increase collagen synthesis and fibrosis. Late gadolinium enhancement studies are positive when hypertrophy is already present (Ho et al., 2010). The myocardial disarray, interstitial fibrosis, and ischemia are also the substrate for the occurrence of arrhythmias.
Phenocopies
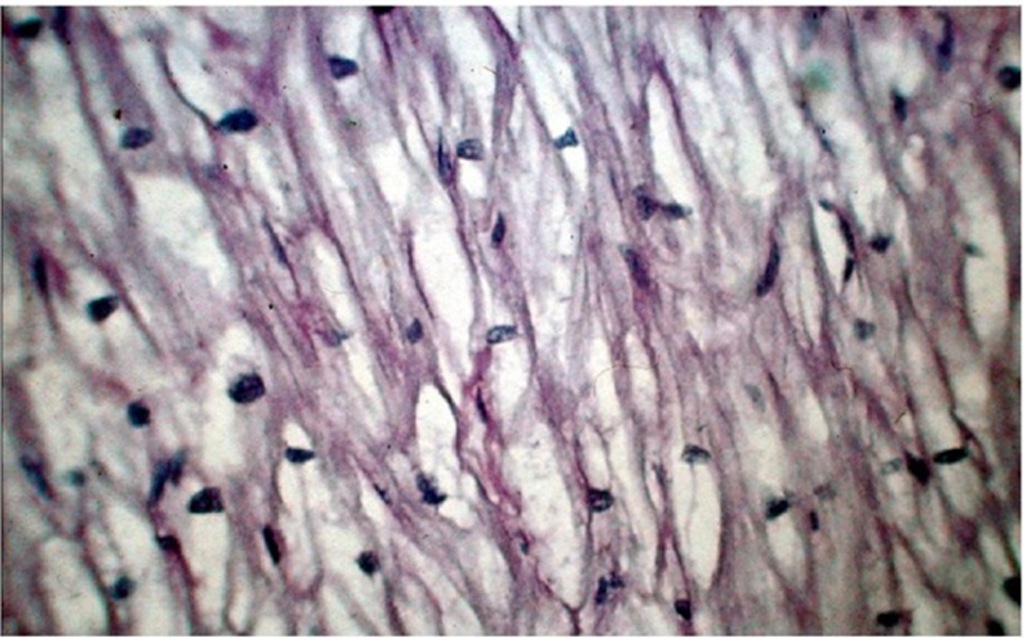
Patients with nonsarcomeric hypertrophic cardiomyopathy are considered to be phenocopies (Table 5), and might have the same pathologic findings as has been reported in some malformation syndromes or neuromuscular disorders like Noonan’s syndrome and Friedreich’s ataxia (Burch et al., 1992; Kawai et al., 2000). However, this is not the case for inborn errors of metabolism like glycogen storage disease where the gross anatomy resembles hypertrophic cardiomyopathy but microscopic examination shows the glycogen deposits in the myocytes without disarray. (Fig. 3) It should be noted that the present definition of phenocopy, according to the Webster’s New World Medical Dictionary in its second acception is: "A person who has an environmental condition that mimics a condition that is produced by a gene". Since the examples just mentioned are genetic in origin, the term phenocopy could be inappropriate but is how these entities have been named for a long time.
Fig. 2. Microscopic view of the myocardium with the typical disarray of hypertrophic cardiomyopathy in an infant who died in congestive heart failure. Myofibers have lost the usual parallel disposition and describe whorls around areas of fibrosis. The disarray is present in the myofibers, among myocytes and in the myofibrils within the myocytes.
Fig. 3. Typical lacework appearance of the myocardium in a patient with type II Pompe’s disease. There is normal alignment of the vacuolated myocardial fibers with glycogen storage.
|
GENE |
PROTEIN |
SYNDROME |
|
TAZ |
Tafazzin (G4.5) |
Barth syndrome/LVNC |
|
DTNA |
a-dystrobrevin |
Barth syndrome/LVNC |
|
LAMP2 |
Lysosome-associated membrane protein 2 |
Danon’s syndrome/WPW |
|
GLA |
a-galactosidase |
Fabry’s disease |
|
AGL |
Amylo-1,6-glucosidase |
Forbes disease |
|
FXN |
Frataxin |
Friedreich’s ataxia |
|
PTPN11 |
Protein tyrosine phosphatase. nonreceptor type 11, SHP-2 |
Noonan’s syndrome, LEOPARD syndrome |
|
RAF1 |
V-RAF-1 murine leukemia viral oncogene homolog 1 |
Noonan’s syndrome, LEOPARD syndrome |
|
KRAS |
v-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog |
Noonan’s syndrome |
|
SOS1 |
Son of sevenless homolog 1 |
Noonan’s syndrome |
|
GAA |
a-1,4-glucosidase deficiency |
Pompe’s disease |
|
PRKAG2 |
AMP-activated protein kinase |
WPW/HCM |
LVNC: left ventricular noncompaction, WPW: Wolff-Parkinson-White, HCM: hypertrophic cardiomyopathy.
Table 5. Genes involved in the production of phenocopies.