By: Breijo-Marquez, FR. MD.
http://breijo-marquez.docvadis.es/
Introduction
Before anything else, is essential to define what is the electrical cardiac systole. Especially when there are so many discrepancies among different authors.
Includes cardiac electrical systole from the beginning of the P wave (atrial depolarization) to the end of the T wave (ventricular repolarization).
Would cover thus the P wave, PR interval, QRS complex, ST segment, T wave.
For other authors, this one only would include from the beginning of QRS complex to the end of the T wave.
There are several changes, especially in its length, that can cause a sudden death in case that they are not adequately diagnosed and, thus, with the properly treated.
Standard values (in length)
| P-wave: 0.06-0.09 seconds in length. |
| PR- interval: 0.12 to 0.20 seconds in length. |
| QRS- complex: 0.06 to 0.10 seconds in length. |
| QT- interval (corrected): 0.40 to 0.44 seconds in length. |
| RR- interval: 0.60-1.00 seconds in length. |
| Normal duration of cardiac electric systole: 35-45% of total duration of the cardiac cycle (R-R interval) |
(The length of cardiac electrical systole is considered normal until reaching 45% of the overall length of cardiac cycle: a greater value is considered as prolonged and lesser is considered as shortened)
The sudden death is defined for most authors as a natural death that happens very instantaneously or within the first hour from the beginning of the symptoms, in a patient with well-known previous disease or without her, but is unexpected totally. Although we do not agree with some nuances of such definition, we will give it as acceptable.
Consequently, any sudden death should be considered either of cardiac origin when the heart is the affected organ, structurally or without macroscopic alterations of its structure. The cardiac problems are the main cause of unexpected death. It is estimated that occurs about 1 case of sudden death for every 100,000 young athletes each year (under 35). Even though exercise is beneficial for health, sport of competition increases the risk of sudden death [Brignole M, et al 2004].
| CARDIAC COMMOTION. |
| CORONARY ARTERY ANOMALY. |
| LEFT VENTRICULAR HYPERTROPHY OF UNDETERMINED CAUSE. |
| MYOCARDITIS. |
| RUPTURE OF AORTIC ANEURYSM. |
| ARRHYTHMOGENIC RIGHT VENTRICULAR CARDIOMYOPATHY. |
| BYPASS CORONARY ARTERY. |
| AORTIC VALVE STENOSIS. |
| ATHEROSCLEROTIC DISEASE OF THE CORONARY ARTERY. |
| DILATED CARDIOMYOPATHY. |
| MYXOMATOUS MITRAL DEGENERATION. |
| ASTHMA. |
| HEATSTROKE. |
| DRUG ABUSE. |
| OTHER CARDIOVASCULAR CAUSES. |
| LONG QT SYNDROME. |
| RUPTURED BRAIN ANEURYSM. |
| CARDIAC SARCOIDOSIS. |
| TRAUMATIC CARDIAC INJURY. |
Table 1. The most frequent causes of sudden death in overall.
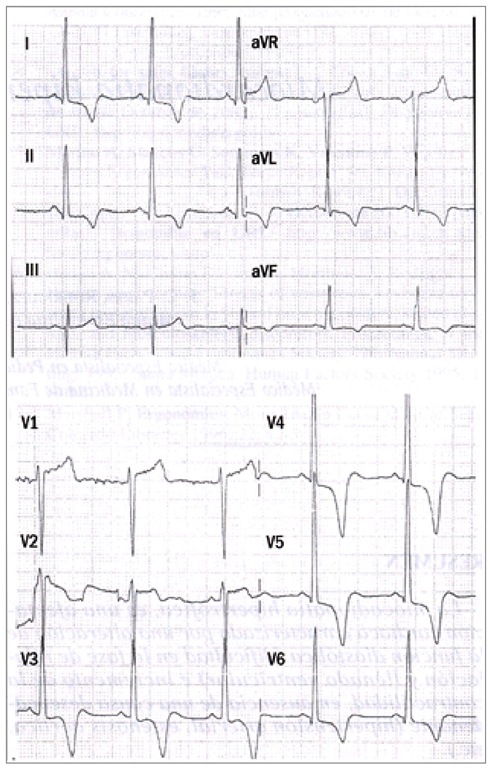
The three most common causes for sudden cardiac death are: 1. Hypertrophic cardiomyopathy (HCM). [Figure 1]:
It is a disease of the myocardium in which a portion of the myocardium is hypertrophied (thickened) without any obvious cause. It is perhaps most well-known as a leading cause of sudden cardiac death in young athletes. The occurrence of Hypertrophic cardiomyopathy is a significant cause of sudden unexpected cardiac death in any age group and as a cause of disabling cardiac symptoms [Richardson P., 1996]. Younger people are likely to have a more severe form of Hypertrophic cardiomyopathy. HCM is frequently asymptomatic until sudden cardiac death, and for this reason, some suggest routinely screening certain populations for this disease [Doerer JJ., 2009].
A cardiomyopathy is a primary disease that affects the muscle of the heart. With Hypertrophic cardiomyopathy (HCM), the sarcomeres (contractile elements) in the heart replicate causing heart muscle cells to increase in size, which results in the thickening of the heart muscle. In addition, the normal alignment of muscle cells is disrupted, a phenomenon known as myocardial disarray. HCM also causes disruptions of the electrical functions of the heart. HCM is most commonly due to a mutation in one of 9 sarcomeric genes that results in a mutated protein in the sarcomere, the primary component of the myocyte (the muscle cell of the heart) [Maron BJ 2010].
While most literature so far focuses on European, American, and Japanese populations, HCM appears in all racial groups. The prevalence of HCM is about 0.2% to 0.5% of the general population [Kuller LH., 1980] 2. Arrhythmogenic right ventricle cardiomyopathy (ARVD). [Figure 2] Arrhythmogenic right ventricular dysplasia (ARVD), also called arrhythmogenic right ventricular cardiomyopathy (ARVC) or arrhythmogenic right ventricular dysplasia/cardiomyopathy (ARVD/C), is an inherited heart disease. ARVD is caused by genetic defects of the parts of heart muscle known as desmosomes, areas on the surface of heart muscle cells which link the cells together [Lahtinen, AM., 2011]. The desmosomes are composed of several proteins, and many of those proteins can have harmful mutations. The disease is a type of non-ischemic cardiomyopathy that involves primarily the right ventricle. It is characterized by hypokinetic areas involving the free wall of the right ventricle, with fibro fatty replacement of the right ventricular myocardium, with associated arrhythmias originating in the right ventricle. ARVD is often found in association with diffuse palmo-plantar keratoderma, and woolly hair, because their genes are nearby and often inherited together. ARVC/D is an important cause of ventricular arrhythmias in children and young adults. It is seen predominantly in males, and 30-50% of cases have a familial distribution.
Fig. 1. ECG is abnormal., 80-90% of cases. Abnormal Q-waves in inferior leads. Increasing the voltage in medium or left precordial (V3-V6). ST segment depression, negative T- waves in precordial leads, middle and left. Less often: Increasing in the left atrium, left axis, Giant negative T waves, atrial fibrillation, ventricular extra-systoles, ventricular tachycardia in severe cases.
Fig. 2. 90% of individuals with ARVD have some EKG abnormality. The most common EKG abnormality seen in ARVD is T wave inversion in leads V1 to V3. However, this is a nonspecific finding, and may be considered a normal variant in right bundle branch block (RBBB), women, and children under 12 years old. RBBB itself is seen frequently in individuals with ARVD. This may be due to delayed activation of the right ventricle, rather than any intrinsic abnormality in the right bundle branch. The epsilon wave is found in about 50% of those with ARVD. This is described as a terminal notch in the QRS complex. It is due to slowed intraventricular conduction. The epsilon wave may be seen on a surface EKG; however, it is more commonly seen on signal averaged EKGs. Ventricular ectopy seen on a surface EKG in the setting of ARVD is typically of left bundle branch block (LBBB) morphology, with a QRS axis of -90 to +110 degrees. The origin of the ectopic beats is usually from one of the three regions of fatty degeneration (the “triangle of dysplasia”): the RV outflow tract, the RV inflow tract, and the RV apex.
Arrhythmogenic sudden death syndrome:
It is a generic name that includes many alterations in cardiac electrical conduction capable of produce instant death.
This syndrome includes all sudden cardiac deaths wherein the cause of death could not be diagnosed, even after the necropsy. It is the cause of more 5% of all sudden cardiac deaths. That is, if we discard the non-cardiac causes and structural heart problems, this problem is denominated as arrhythmogenic sudden death syndrome from a generic form. As the diagnostic techniques are being more appropriate each day, these numbers grows exponentially [Strickberger SA., 2006].
Here, would be included all events from our topic proposal : Alterations in electrical cardiac systole and its impact on sudden cardiac death.
Other disorders in electrical cardiac systole as cause for sudden cardiac death
As we have said previously, the electrical cardiac systole originates from the beginning of the P wave (atrial depolarization) to the end of the descending branch of the T wave (ventricular repolarization). Are included, therefore, the succession of P-QRS-T and its corresponding intervals and segments: PQ, ST and QT. The mathematical possibilities in the variation on length of electrical systole of the heart may be several. It is well documented and demonstrated that such changes in length can cause that be more vulnerable and unstable all myocardial cells, and can also cause serious cardiac arrhythmias, several syncope episodes and even sudden death for this motive. Even today, many of these disorders are poorly understood and, too many times, its clinical manifestations are categorized as “episodes of epilepsy”; other times (most) are classified within a “common sack” called “channelopathies”, when -actually- is the alteration from electrical cardiac systole the true etiology of them.
All these disorders can cause syncopal episodes and a sudden cardiac death.
The measures and lengths of the different components of electrical cardiac systole, considered for most authors as normal are these:
PR-interval: 0.120- 0.200 seconds.
QRS complex: 0.08-0.120 seconds.
QT-interval (corrected): 0.350-0.450 seconds. (Here, there is much disagreement among different authors). The most used methods for QT interval correction, since it is frequency-dependent, are Bazett, Fridericia.
When the PR-interval is lesser than 0.120 seconds, we call it a short PR-interval. In contrast, when is greater than 0,200 seconds, we call it a first-degree AV block. When the QRS complex is lesser than 0.08 seconds, we call it “narrow QRS” but when is greater than 0,120 seconds, we call it “wide QRS”. Likewise, when the corrected QT- interval length is lesser than 0,350 seconds, we call it Short QT- interval and when is greater than 0,450 seconds, we call it a Long QTc- interval.
It is clear that there may be, in the same ECG recording, a combination of them all. Some of these disorders, we will explain briefly below.
Wolff-Parkinson-White’s syndrome (WPWS)
Wolff-Parkinson-White syndrome (WPWS) is a congenital heart disease (PRKAG2. Genetic map 7q36) characterized by a premature ventricular depolarization caused by an abnormal atrioventricular accessory pathway, between the atria and ventricles, known as Kent’s bundle. However, even today, is called into question the real cause of Wolff-Parkinson-White, there are some authors who believe that, PRKAG2 mutations, are caused by a glycogen storage cardiomyopathy associated with WPWS, because the overwhelming majority of accessory pathways occur in individuals without structural heart disease, and probably without this mutation. The pathogenesis of accessory pathway formation in PRKAG2 may be completely different, and some authors believe it is due to an inflammation of myocardial cells that occur in the atrial-ventricular connections [L. Wolff., 1930].
In fact, do not even know if the accessory pathways are mediated genetically or due to environmental exposures or randomly.
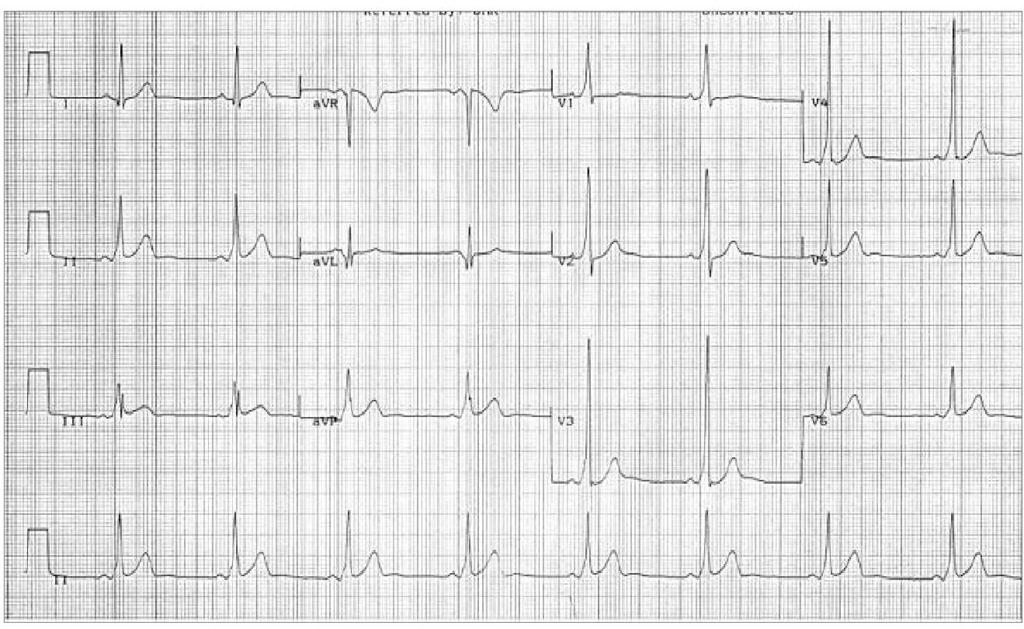
A short PR interval, a delta wave, a wide QRS complex (greater than 120 ms) and, occasionally, alterations in the ventricular repolarization are its main electrocardiographic characteristics on the ECG. Its incidence varies between 0, 1% and 3% in the general population.
It is essential to achieve the right differential diagnosis between:
• Wolff-Parkinson-White’s syndrome or real ventricular pre-excitation.
• Lown-Ganong-Levine syndrome or accelerated atrioventricular conduction.
• Mahaim’s syndrome.
• “Short PR alongside short QT” intervals in the same person.
Typical ECG image of the Wolff-Parkinson-White
In this context of ECG recording, that has a normal heart rate, a short PR interval, a delta-wave and an early ventricular repolarization can be seen.
3.2 Lown-Ganong-Levine syndrome (LGL)
This syndrome was described in 1952 by Lown, Ganong, and Levine, forming the famous now used to describe it. It is considered a preexcitation syndrome [Lown B, Ganong WF, Levine SA., 1952].
We now know four types of pre-excitation syndrome:
• Wolff-Parkinson-White or ventricular preexcitation true.
• Lown-Ganong-Levine or accelerated atrioventricular conduction.
• Short PR alongside short QT” intervals in the same person.
• Mahaim Syndrome.
LGL is a disease entity that is included within the more general condition called Short PR-Interval).
Etiology
• Acquired .
• Congenital :
• Inherited.
• Not inherited.
The familial form is inherited, as an autosomal dominant genetic trait has been associated with the PRKAG2 gene that encodes the activated AMP protein kinase, responsible for transport and store energy from the heart. A mutation in this gene could explain the susceptibility of the heart to the crises of tachycardia. Mutation has been identified on the long arm of chromosome 7 (7q34-q36).
The Lown-Ganong-Levine may affect approximately 1 in every 50,000 people. Several structural abnormalities have been proposed as the possible basis for LGL, including the presence of James’s fibbers, Mahaim’s fibbers, Brechenmacher and underdeveloped anatomic sinus node (hypoplastic).
Each of these fibbers can only be identified histologically.
Thus, unless other studies demonstrate definitive- structural or functional -abnormalities, the diagnosis of LGL remains a clinical diagnosis.
In the absence of significant structural heart disease, the mortality rate appears to be very low. Patients may present with an acute episode of tachycardia or a history of symptoms suggestive of paroxysmal tachycardia. In diagnosis is necessary to make:
1. A standard test for tachycardia, including an ECG to document the rhythm.
2. Serum electrolytes, calcium, magnesium levels, and levels of serum thyroid hormone-stimulating hormone (TSH). Lithemy.
3. History suggestive of recurrent paroxysms of tachycardia,
4. A Holter monitor or event recorder may be useful to document the rhythm during acute symptomatic episodes.
5. An ergometric study.
6. In rare cases, an implantable monitor for pace may be helpful.
7. Family History. (Screening).
Differential diagnosis with Wolf-Parkinson-White
Although apparently similar, there are differences, which, in our opinion, are critical with respect to drug treatment elective. The key differences are:
- The LGL is a PR- interval shortened due to, the presence of accessory pathway, prevents the AV node but normal QRS because the accessory pathway (James fibbers) binds directly to the sinus and depolarizes the ventricles not directly, but does so by typical pathway, by the Hiss-Purkinje system.
- Not displayed “Delta waves -” in D1, aVL, V5 and V6.
- The QRS complexes tend to be narrow because there is usually no interventricular conduction disturbance.
- It is not be as frequent the association of atrial fibrillation during concomitant crisis.
Prognosis
No studies have shown an increased risk of sudden death or reduced survival for patients meeting the criteria for the diagnosis of LGL.