Cardiopulmonary reflex
Despite the great importance of baroreflex in controlling arterial pressure, several investigations have demonstrated that the neural reflex of circulation also depends on cardiopulmonary reflex.
Cardiopulmonary receptors are found in low pressure portions of the circulation, such as walls of the atria and pulmonary arteries. These mechano-sensitive receptors are activated by the distension of the vessels walls, responding to changes in central blood volume (Thomas, 2011). The impulses arising from these receptors exert a tonic restraint on cardiac function and contribute to the physiological control of circulation. Cardiopulmonary reflexes are stimulated not only by changes in cardiac filling pressure but also by chemical agents, such as prostaglandins and serotonin (Vasquez et al., 1997). The cardiopulmonary fibers converge to the same pool of central neurons as the baroreceptors and act in a similar way (Spyer, 1990). Therefore, increased discharge of cardiopulmonary vagal afferent C fibers results in reflex enhancement of parasympathetic activity and decreased sympathetic outflow, leading to bradycardia, hypotension and apnea, also known as the Bezold-Jarisch reflex (BJR) (Kashihara, 2009). In addition, increased discharge of the cardiopulmonary receptors diminishes renal sympathetic outflow and pituitary release of vasopressin, thereby decreasing Na+ and water reabsorption by the kidneys, increasing urine volume, and reducing blood volume. As changes in blood volume affect cardiac output and arterial pressure, this provides an additional mechanism by which the cardiopulmonary reflex contributes to blood pressure regulation (Thomas, 2011).
Interestingly, cardiopulmonary reflex may exert a tonic inhibitory influence in the arterial baroreflex sensitivity (Abboud and Thames, 1983). In pathological conditions, such as acute myocardial infarction, the reduction in baroreflex sensitivity could be explained by an increase in cardiopulmonary reflex sensitivity (Lacerda et al., 2007). Furthermore, the BJR activation might cause sudden cardiac death during ischemic injury (Robertson et al., 1985), since the overactivation of cardiopulmonary reflex together with baroreflex blunting might cause severe bradycardia and hypotension, placing the patient’s life at risk due to the magnitude of sympathetic inhibition and vagal activation. These data demonstrate that the interplay between baroreflex and cardiopulmonary reflex may exert an important role in the progression of cardiovascular diseases and arrhythmias generation.
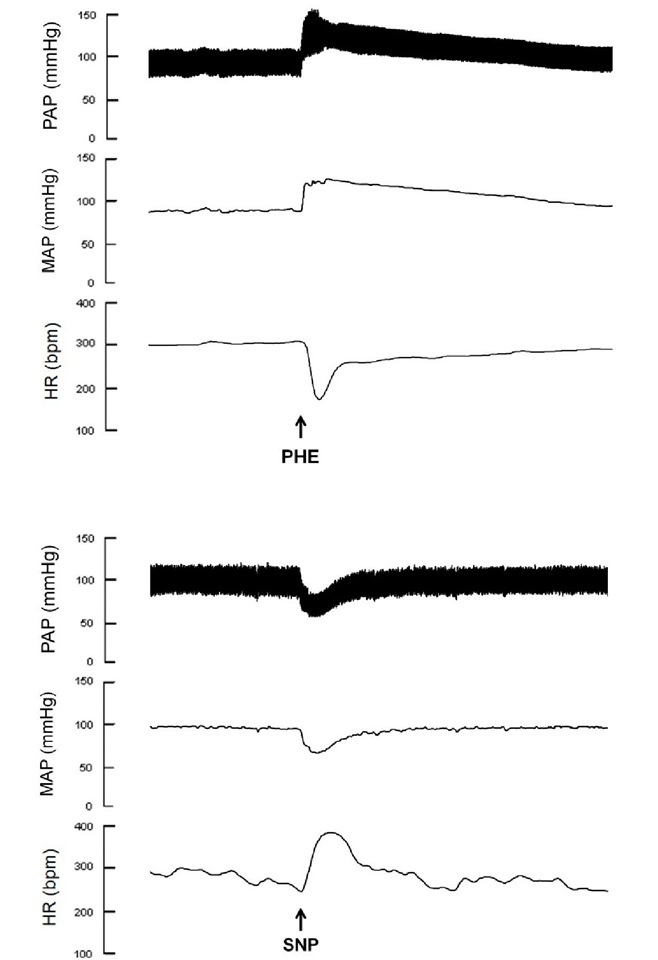
Fig. 2. Typical recordings of baroreflex evaluation in anesthetized rats. The phenylephrine-induced increase in arterial pressure leads to reflex bradycardia (upper panel) and the sodium nitroprusside-induced decrease in arterial pressure results in reflex tachycardia (lower panel).
Experimentally, the cardiopulmonary reflex function can be evaluated through phenylbiguanide injections. Figure 3 shows typical recordings of changes in arterial pressure and HR during cardiopulmonary reflex test.
Fig. 3. Typical recordings of cardiopulmonary reflex evaluation in anesthetized rats. The activation of cardiopulmonary receptors is achieved by intravenous phenilbiguanide injections.
The peripheral chemoreflex is considered one of the main mechanisms of control of the ventilatory responses to the changes in arterial O2 and CO2 concentrations. The peripheral chemoreceptors located in the carotid and aortic bodies, with afferents to the respiratory center in the medulla oblongata and the NTS, respond primarily to hypoxia (Guimaraes et al., 2009). These chemo-sensitive receptors constantly receive information of arterial pO2, pCO2 e pH through a thin artery originated in the middle of the bifurcation of the common carotid artery that maintains these cells in close contact with blood gases (Vasquez et al., 1997). Increases in the firing rate of these neurons lead to a simultaneously activation of sympathetic outflow to blood vessels and increased vagal activity to the heart (Kara et al., 2003). Therefore, the excitation of the peripheral chemoreceptors produces an increased minute ventilation, systemic vasoconstriction and hypertension. The primary HR response to chemoreceptor stimulation is a parasympathetic mediated-bradycardia, but this mechanism is usually apparent only in the absence of ventilation. In the presence of the normal ventilatory response to hypoxia, tachycardia is generated by a lung inflation reflex that inhibits vagal outflow to the heart (Marshall, 1994). It is interesting to notice that, if blood pressure is within its normal range, the chemoreflex does not evoke a powerful cardiovascular response because of the predominant effect of the arterial baroreflex.
However, if blood pressure is low, generally below 80 mmHg, activation of the chemoreflex potentiates the vasoconstriction evoked by the baroreflex and helps to restore blood pressure to normal (Thomas et al., 2011).
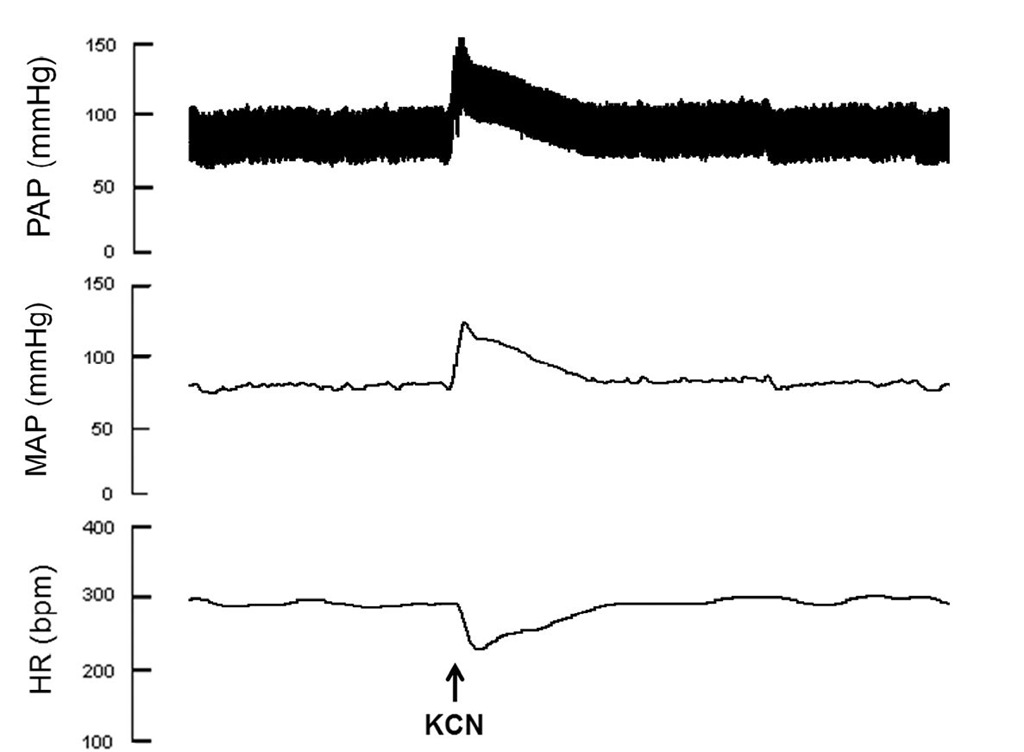
The role of chemoreflex in cardiac arrhythmias have been already demonstrated. Patients with survived ventricular arrhythmias show significantly decreased chemoreflex sensitivity (Hennersdorf et al., 1997). The chemoreflex sensitivity is also considered as a marker of increased risk for ventricular tachyarrhythmias, since it shows a high positive predictive power in patients with prior myocardial infarction and who previously survived ventricular tachyarrhythmias (Hennersdorf et al., 2002). Central sleep apnea, which is associated with absent respiratory effort and results from instability in the chemoreflex control of breathing, is thought to predispose to cardiac arrhythmias generation (Leung et al., 2009). Experimentally, the chemoreflex function can be evaluated through potassium cyanide injections. Figure 4 shows typical recordings of changes in arterial pressure and HR during chemoreflex test.
Fig. 4. Typical recordings of chemoreflex evaluation. The activation of chemoreflex is achieved by intravenous potassium cyanide injections.
Humoral control of HR
Several investigations have demonstrated that humoral systems can play a pivotal role in maintaining cardiac electric activity homeostasis and changes in their production and/or action pathways may contribute to various disorders in cardiac excitability. Additionally, the humoral systems can also modulate the autonomic nervous system and cardiovascular reflexes, demonstrating the importance of these substances in cardiovascular functioning.
Estrogen
The sexual hormones are related mainly with the control of reproductive function, however they can also modulate the cardiovascular function. Estrogen is the main female sex hormone in both humans and animal models. It is produced in the granulosa cells of the ovarian cortex through the conversion of androgen precursors by the aromatase enzyme, which in turn is modulated by the hormonal hypothalamic-pituitary axis (Filicori, 1986). The protective effects of estrogen on cardiovascular function have been already demonstrated by several investigations. Indeed, estrogen replacement therapy reduces the incidence of coronary artery disease (Rowland & Fregly, 1992; Farhat et al., 1996). Corroborating these data, Moyses et al. (2001) showed that estrogen treatment in ovariectomized female rats restored coronary vasodilation produced by serotonin in isolated hearts, and a bolus injection of 176-estradiol elicited a transient vasodilatory response in male and female normotensive (Santos et al., 2004) and spontaneously hypertensive rats (Santos et al., 2010). It has been already demonstrated that estrogen levels are also related with the development of cardiac arrhythmias. During the menstrual cycle, estrogen levels rise and fall in women and these fluctuations are related with more frequent episodes with a longer duration of supraventricular tachycardia (Rosano et al., 1996). During perimenopause there is a marked decrease in ovarian estrogen production that is associated with an increase in HR (sinus tachycardia) and an enhancement in the frequency of palpitations and non-threatening arrhythmias, such as premature ventricular contractions (Rosano et al., 1996; Asplund and Aberg, 2003). During menopause a further decline in estrogen occurs and this event is associated with irregular heartbeats, palpitations, spasmodic chest pain and nightmares in women from 40 to 64 years old (Asplund and Aberg, 2003). Corroborating this data, hormonal replacement therapy (HRT) may decrease palpitations and other symptoms such as hot flashes, insomnia, and sweating (Grady et al., 2002). On the other hand, the Heart and Estrogen/Progestin Replacement Study (HERS) found no benefit to reduce cardiovascular events in women on HRT, which may even increase risk of thromboembolism during the first year (Grady et al., 2002). HRT has also being associated with lengthening the QT interval, although the relevance of this finding is not known (Gokce et al., 2005). Therefore, more investigations are necessary to better elucidate the benefits of HRT in preventing cardiac arrhythmias generations.
The mechanisms by which estrogen may affect the development of cardiac arrhythmias include changes ion channels expression and/or activity. Most of the studies demonstrate that estrogen exerts antiarrhythmic effects, possibly by acting the L-type Ca2+ channels, contributing to its cardioprotective actions (Nakajima et al., 1999). Ulrich et al (2007) demonstrated that estrogen inhibits ICaL through direct interactions of the steroid with the channel protein in a rate dependent way, leading to a decreased contraction. However, estrogen can also upregulates the sodium-calcium exchanger (NCX1) through a genomic mechanism mediated by estrogen receptors (ER), contributing to the enhanced propensity to early after depolarizations in female hearts (Cheng et al 2011).
It is well established that estrogen can cross the blood brain barrier and be accumulated in regions of the brain to bring about changes in neural activity, including in autonomic functions (Lee and McEwen, 2001). This modulation may occur via activation of ERs, since ER mRNAs expression have been identified in central areas controlling cardiovascular function such as, NTS, CVLM, RVLM and IML (Spary et al, 2009).
Several studies have demonstrated the effects of estrogen on cardiovascular reflexes. In ovariectomized female rats, intravenous estrogen supplementation significantly reduced sympathetic tone within 30 minutes and significantly increased parasympathetic tone within 5 minutes of administration (Saleh and Connel, 2000). Corroborating these findings, Flues et al (2010) demonstrated that ovariectomized rats supplemented with 17P estradiol presented an exacerbated vagal tonus when compared to ovariectomized rats. This study also showed that ovarian hormones deprivation induced a higher sympathetic activity to the heart. Additionally, Minson et al (2000) reported an increase of baroreflex sensitivity (BRS) in phases of menstrual cycle with estrogen preponderance. An enhanced BRS is associated with an increase in parasympathetic and/or a decrease in sympathetic tone (Rovere et al, 2000), and the degree of BRS depression is significantly correlated to an increased likelihood of cardiac arrhythmogenesis (Saleh et al, 2003). Taken together, those data indicate a beneficial effect of estrogen in autonomic balance and in arrhythmias prevention.