Congenital cardiac arrhythmia
LQTs is charactered by the prolongation of QT interval on ECG of the patients. Loss of function in Kv11.1 caused LQT2. Defective synthesis of mutations contained the premature termination codons is maybe the one fourths of all mutations of hERG channel (http: //www. fsm. it/cardmoc). Otherwise, hERG mutation of R534C display an increased open probability expressed in Xenopus laevis oocytes whereas in clinically the mutant induces the gain of function. Therefore, it is importance of detecting the mutations in mammalian system.
Gain of function mutation in KCNH2 cause increasing of currents amplitude IKr which lead to shorten the action potential duration and in final to decurtate the QT interval on ECG. Some patients with SQT will be healing well in the future through the regulation of themselves. However, if the cardiac action potential is persistent shorten and produce diminishing of refractory period between the continual bisaction potential. At last, SQT may be get worse the atrial fibrillation and sudden death or syncop.
Acquired LQTS
Comparision with congenital LQTS, acquired LQTS is more common cause of TdP. Lots of factors can induce to form acquired LQTS, such as myocardial ischemia, electrolyte disturbances, bradycardia and so on. Of the most important factors is drug. Accordingly, the drug-induced LQTS is equal to the acquired LQTS in most case. However, acquired LQTS and drug-induced LQTS is essential two different concepts.
Many kinds of drug can induce the acquired LQTS, for instance antiarrhythmia drug, antibiotic, antihistamine and so on. Atiarrhythmia drug quinidine is a relatively frequent side effects, caused 2-9% of treated patient induced RdP. Other drugs induced TdP is less than antiarrhythmia drugs. The compounds (dofetilide, sotalol and ibutilide), predictable designed to block cardiac repolarizing currents, can induce the prolongation of the OT interval which unfortunately arises as a side effect of the compound treated for non-cardiac diseases. Therefore, the compound in already marketed drugs will be withdrawal or restriction. In order to the expenses of the pharmaceutical companies, it is now common practice to screen for compound for hERG-channel activity early during preclinical safety assessment. However, in clinical the blockage of hERG can counteract by blocking of L-type of calcium channel.
Most of drugs which can induce acquired LQTS can also block of hERG channel. Contrast to other Kv channel, the hERG is unusually susceptible to blockage by drugs is unknown, suggesting that it has a unique binding site. In order to find the interact sites hERG with the blockers, an ala-scanning mutagenesis approach is used. Mutations of two polar residues (Thr 623 and Ser 624) located at the base of the pore helix and mutations two aromatic residues Tyr652 and Phe656 located in the S6 domain of hERG has vital roles in combination with the compounds. The side chains of all four residues are oriented toward the large central cavity of the channel and can block the transmembrane pass of potassium ions by combining with the blocker.
Future perspectives
Over the past 16 years a great deal of discovery of hERG channel has been detected but there is still much to explore about the channel.
Kv7.1
Kv7.1 (also known as KCNQ1, KvLQT1) is the a-subunit of a voltage-gated potassium channel cloned in 1996 by Wang and co-workers using linkage analyses of LQTS1 patients and expressed in several tissues including cardiac myocytes and epithelial cells. The most important roles of KCNQ1 channels are repolarization of the cardiac tissue following an action potential. In cardiac myocytes, the KCNQ1 subunit assembles with the KCNE1 P-subunit (minK) to form a channel complex. The channel complex of KCNQ1/KCNE1 produce the delayed rectifier current IKs, which is partly responsible for terminating the cardiac action potential during phase 2. Up to now, there are nearly three hundred mutation of KCNQ1 have been detected. Most of the mutations produce the loss of function of KCNQ1, lead to the LQTS (http://www.fsm.it/cardmoc/), a most kind of cardiac arrhythmia characterized by prolongation of QT interval in electrocardiogram, syncope and sudden death. Only a few gain-of function mutations have been verified and have correlate with the atrial fibrillation or the short QT syndrome (SQTs).
Based on the molecular mechanism of altering of KCNQ1 currents, mutations of the channel are divided into impaired trafficking, impaired voltage dependence, impaired selectivity and impaired tetramerization. For the majority of these three hundred KCNQ1 mutations, little is known about the molecular mechanism producing to the pathologies or limited to which of the categories. Therefore, there are many unknown knowledge about the three hundred KCNQ1 mutaions and make further progress in the future with the application of new technology of structure prediction in study.
Structure and electrophysiological characters of KCNQ1
As a member of Kv potassium channel, KCNQ1 channel show a high similarity to voltage-gated potassium channels of the Kv type which assemble a tetramer, with each subunit of KCNQ1 contained S1-S6 trans-membrane. Different from other Kv members, KCNQ1 often form heterotetramer with auxiliary subunits contained one-transmembrane in vivo. To date, there are five member of KCNE family have been detected and in Xenopus laevis oocytes or mammalian system the a subunit of KCNQ1 channel can combinate with any one of them to form miscellaneous kinds heterotetramer with distinct kinetic characters of channel such as activation, inactivation or deactivation and so on. Accordingly, a subunit of KCNQ1 channel has been check in lots of tissues in the body and with different physiological characters.
In the full-length human KCNQ1 gene, 16 exons constitute of KCNQ1 with the very GC-rich 5′-end. The translated protein is composed of 676 residues and has six transmembrane domains S1-S6, a pore loop with a typical potassium-channel pore signature sequence (GYGD), and intracellular NH2 and COOH terminals covering 122 and 322 residues, respectively. To date, with the exception of KCNQ1, there are other four members in the KCNQ family have been detected, such as KCNQ2-5. Comparision with other members of KCNQ family, KCNQ can not assemble a hetertetramer with other members and just forming a homotetramer only with themselves.
As a voltage-gated potassium channel, KCNQ1 was activated by decreasing of depolarization. And similar the other voltage-gated potassium channel, the voltage sensor is located in the S4. However, mutations in the linker of between S4 and S5 still have effect on activation of the KCNQ1 channel. When KCNQ1 channel are fully open at the positive potential followed by a strikingly repolarization produces a hook currents which represent a fraction of KCNQ1 channel inactivation. Because the channel will be open again from the closed state. Researches show that the five transmembrane and the pore part of each subunit have vital roles in the inactivation of KCNQ1 channel.
Regulation of KCNQ1 channel activation and inactivation
As mentioned above, the KCNQ1 subunit and the ancillary subunit KCNE1 collect together to form the currents of IKs in cardiac tissues. The KCNE1 subunit has important roles in regulation of kinetical properties of KCNQ1 channel. For example, the currents formed by KCNQ1 subunit alone is activated rapidly whereas ones formed by coexpressed of KCNQ1 and KCNE1 is activated very slowly. In addition, the presence of KCNE1 produce a large increase in the macroscopic KCNQ1 currents, a positive shift of voltage-dependence curves, slowing of the activation and deactivation and almost of absent of inactivation. Some researches have shown that the distinguished increase of the magnitude of currents of KCNQ1/KCNQ1 complex is owing to increase the single channel conductance for four to sevenfold and almost eliminate the inactivation the channel by KCNE1 subunit. As for the exact combination of KCNQ1 and KCNE1 subunit, there are distinct views about it. Some results display that KCNE1 lines to the conductance pathway. On the contrary other results show the combination site is out of the conductance pathway. Van Horn proposes a Q1/E1-TMD model, a new model to elucidate the interaction of protein-protein about KCNQ1 and KCNE1 in recent researches. The emphasis of the new model is on the KCNE1 transmembrane domain (also called TMD). It is generally accepted that in closed state the S4-S5 linker interact with the C-end of S6 from another subunit to lock it in the closed configuration. In response to depolarization, the change of conformation of S4 voltage sensor, the S4-S5 linker pull off and deviate from the S6 inducing the channel open. The Q1/E1-TMD model consider that the C-terminal end of KCNE sits on the end of the S4-S5 linker while simultaneously N-terminal end makes extensive (and presumably adhesive) contacts in the cleft between the voltage sensor and pore domains of the channel. Therefore, during the transition of the channel from closed to open state, the presence of KCNE1 TMD will interfere with the S4-S5 linker deviating from the S6. Uniformly, because of the KCNE1 presence, transition state open to close become very slow and is help for maintenance of the open state of the channel.
Besides the ancillary subunit of KCNE1, there are other members (KCNE2-5) in the family. It is interesting that all ancillary subunits can co-assemble with KCNQ1 channel in different tissue and alter the kinetic characters of KCNQ1 channel. In cardiac tissue, besides of KCNE2, KCNE2 is another important ancillary subunit. KCNE2 (also named Mirp1), originally described as a ancillary of the ether-a-go-go-related gene 1 (ERG1) potassium current, was later found to change the KCNQ1 potassium current though drastically changing the gating properties. Otherwise, in organs such as stomach and intestine, Moreover, the mRNA of KCNE4 and KCNE5 has been detected in the heart. They may be has vital roles in maintaneine of the ordinary function of the heart. However, there is no relative report about it.
Functions of KCNQ1 channel in heart
Currents of IKs formed by KCNQ1/KCNE1 have slow activation, whereas IKur and IK1 constituted by ERG1 and Kir2.x, respectively, have rapide activation kinetics. The three repolarizing potassium currents together have been called the repolarization reserve because to some extent they can substitute for each other. However, in the fast heart beat, only IKs currents are upregulated by phosphorylation and by current accumulation due to slow deactivation. In addition, In heart tissue, distribution of KCNQ1 through the cardiac wall is also inhomogeneous and the expression of KCNQ1 is less in medmyocardium than epi- and endomyocardium.
The cardiac function of KCNQ1 and its accessory subunits is emphasized by the functional impact of numerous mutations in these proteins (http://www.fsm.it/cardmoc/). Mutations in KCNQ1 causing loss of function by trafficking defective, assembly defective, or single channel conductance lead to prolonged action potentials and LQTS. A domain located near the COOH terminal (residues 589-620) is responsible for this assembly specificity, and deletion of a part of this domain leads to an impaired assembly of the channel complexes followed by mistrafficking. Mutations in ancillary subunits such as KCNE1 and KCNE1 also cause LQTS4 and LQTS5, respectively.
KCNQ1 mutation (S140G), as a gain of function mutation, is detected in a family with arterial fibrillation inherited as an autosomal dominant way through four generations. The mutation shortens the action potential through increasing the currents of IKs. Similarly, a gain-of-function mutation in KCNE2 (R27C) increasing the activity of the KCNQ1/KCNE2 channel has also been implicated in atrial fibrillation.
Acquired LQTS is predominantly found when the patients take the blocker of hERG channel as medicine. Because the currents of IKr are blocked, the repolarization reserve is decreased and the disperation of repolarization is leaded to a further increase due to the inhomogeneous distribution of KCNQ1 in heart wall.
Kv4.3
Kv4.3 is formed the rapid activated currents Ito (encoded by KCND3) which is a voltage-dependent, 4-aminopyridine (4-AP) sensitive, calcium-independent K+ current (Ito). Ito have been detected in human artial and ventricular myocytes and is responsible for the early rapid depolarization (at phase 1) so determining the height of plateau. Therefore, Ito will influence of other ion channel activation such as the L-type calcium channel and the delayed rectifier channel (KCNQ1). Distribution of Kv4.3 is heterogeneous through the cardiac tissue. For example, Ito density in atrial tissue, Purkinje fibers, epicardial and midmyocardial (M) cells is higher than in the endocardial cells. The prominent epicardial Ito conduce to the depression of epicardial in ischemia and to the progress of a significant dispersion of repolarization between normal and ischemic epicardium, between epicardium and endocardium.
Regulation of Kv4.3
Kv4.3 can be blocked by many compounds but which bind with the channel either at open state or at close state. It has been raised that blocker of Ito prolong the action potential duration in atria or in ischemic ventricular tissues. However, blockage of Ito subsequently changes the other potassium channel underlying during repolariztion of cardiac action potential. Reduction of Ito magnitude can shorten the duration of ventricular action potential. Therefore, it is still unclear that the exact role in control human cardiac action potential. Channel properties of Kv4.3 is modified by the phosphorylation, mediated by protein kinase A (PKA) and C (PKC) through altering the channel kinetic (activation, inactivation or single channel conductance) and the expression of active channel in the membrane. Decrease in Ito by PKC attributed to enhance the inactivation and step down the time of deactivate of the channel Kv4.3. a-adrenergic agonists reduce Ito magnitude in rat ventricular myocytes and oppositely the P-adrenergic agonists has no effect on Ito currents.
Functions of Kv4,3 in heart
Heart failure, cardiac hypertrophy and myocardial ischemia and infarction decrease the magnitude of Ito resulting in the prolongation of action potential. The degrade in Ito in heart failure may be adaptive in the short-term because increased depolarization during the cardiac cycle means that more time is available for excitation-contraction coupling, which moderate the decrease in cardiac output, however it becomes maladaptive in the long-term, because a prolongation of the APD may contribute to arrhythmogenesis, either by causing inhomogeneous repolarization or by increasing the likelihood of early afterdepolarizations. On the contrary, it is proved that up-regulation of Ito in cardiac hypertrophy and in cardiac myocytes after induced myocardial infarction. Increase in Ito presents as a protector moderating the excessive prolongation of action potential duration and Ca2+ inflow to minimize the incidence of ventricular arrhythmia. In addition, the patients with chronic atrial fibrillation decrease the currents of Ito and downregulate the mRNA.
Kv1.5
Ikur currents in human atrium are formed by the a subunit (Kv1.5) and P ancillary subunit (KvP1.2). The features of IKur, as outward rectifired currents, are activated rapidly in the plateau range and inactivation slowly. Interestingly, currents of IKur just have been detected in human atria rather than cardiac ventricle. Therefore, currents of IKur have vital roles during the atrial repolarization. There is a huge difference of Kv1.5 from other ion cardiac channel. Distribution of Kv1.5 is homogeneous across the atrial wall.
Regulations of Kv1.5
hKv1.5 can be regulated by both PKA and PKC. One consensus site in Kv1.5 for phphosphorylation by PKC is located on the extracellular S4-S5 linker and 4 consensus sites for PKA is located in the N- and C-terminal domains. Isoproterenol and adenylate cyclase both increase the magnitude of IKur and the increase can be counteract by PKA inhibitor. Otherwise, propranolol and phenylephrine decrease the amplitude of IKur moderating by the PKC inhibitor. Accordingly, P-adrenergic stimulation enhances the currents of IKur by PKA. Oppositely, a-adrenergic stimulation inhibits IKur currents by PKC. Human thrombin or rat 5-HT1c receptors inhibits the currents of IKur by increasing phospholipase C (PLC). Moreover, the Scr tyrosing kinase inhibits the hKv1.5 by phosphorylation of the N-terminus proline-rich sequences mediated by SH3 domain of the tyrosine kinase.
Functions of IKur in heart
IKur is relatively insensitive to TEA, Ba2+ and class III antiarrhythmics of the methanesulfonanilide group. Antiarrhythmia drug is often weak bases that predominant cationic ion at pH7. At the channel open state, the cationic ion can bind with the pore and/or selective filter domain of the channel leading to the blockage of the channel. The binding site for some drugs is existed at the external mouth of the channel pore formed by the P loop and adjacent S5-S6 segments.
Because of the Kv1.5 just located in atria, the channel is a promising target for the development of new safe antiarrhythmic drugs to prevent atrial fibrillation and without a risk of ventricular proarrhythmia. However, In patients in chronic artrial fibrillation, the action potential duration in atria is significantly prolongated due to both blockage of Ica,L and IKur. Accordingly, it is not expected what will be happened by use of IKur blocker to treat the patient with chronic AF. In a rat, rapid atrial pacing just immediately and transiently increases the mRNA of Kv1.5 rather than the ones of KCNQ1 and hERG. It shows that Kv4.3 at least in part contribute to the rapid shortening of the atrial refractoriness at the onset of AF. Therefore, the selective blockers of IKur counteract the shortening of atrial action potential duration at rapid rate state. From mentioned above, application of IKur selective blocker in clinical is a challege job in the future.
Kir2.1 and Kir3
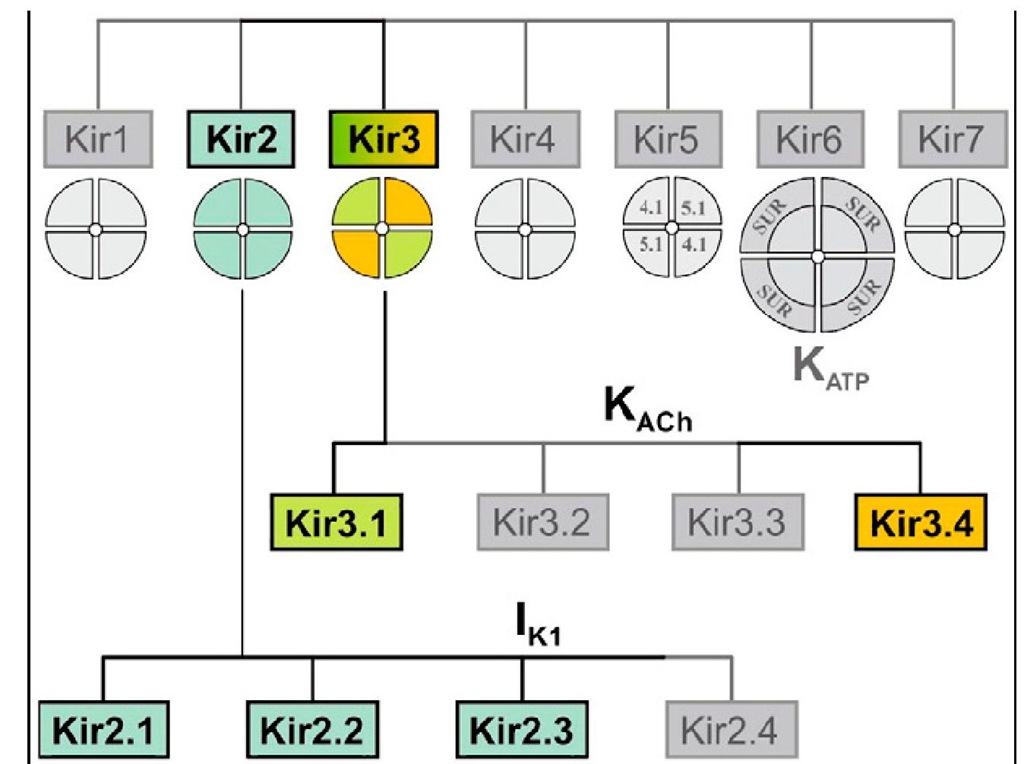
Inward rectifiers (Kir) is composed of a large family of potassium channel. Among them, only two subfamilies (Kir2 and Kir3) share great structural similarity and underlie classical ‘strong inwardly rectifying currents’ originally observed in skeletal and cardiac muscle. In cardiac tissue, there are only two similar types of these currents: (1) IK1, as a constitutively active Kir current, is more prominent in ventricular tissue, and (2) IK,Ach, as a receptor-activated Kir current, is more prominent in atrial tissue, as well as in SA node and AV node. There are two common features of the kir2 and Kir3. one is a strongly voltage-dependent decline of potassium conductance upon membrane depolarization producing a characteristic region of so-called ‘negative slope’ conductance. Another unique property of Kir currents is the unusual dependence of rectification on extracellular K+. In order to comprehend the two characters of Kir channel, firstly to fully the molecular basis of the channels.
Fig. 4.The family of inward rectifier potassium channels. All members of this family share significant structural similarity, but only Kir2 and Kir3 subfamilies represent channels carrying classical strongly rectifying currents.
In human heart, the distribution of IK1 and IK,Ach has distinct region. IK1 is more prominent in the ventricles, including Purkinje myocytes. IK,Ach has generally an opposite distribution to that of IK1. It is more prominent in the atria than in ventricles. Similarity, the current density of IK1 and IK,Ach may vary across the ventricular or atrial tissues, distinctively. About the subunit composition of IK,Ach, under normal conditions native IK,Ach channels are heteromers of Kir3.1/ Kir3.4 subunits. However, recent data suggest that Kir3.4 subunit alone has similar function with the native IK,Ach. Comparision with IK,Ach, IK1 is formed by coassembly of the Kir2.1.x subfamily of proteins (Kir 2.1, 2.2, and 2.3) with Kir2.1 the most abundant subtype in ventricular tissue.