Cardiac sodium channelopathies
To date, more than 150 mutations in SCN5A have been reported, the vast majority of them caused either LQTS3 and Brugada syndrome. Some patients with LQTS can be healing well whereas most of them may increase risk for sudden death due to ventricular tachyarrhythmias, in particular torsades de pointes. The character of LQTS3 is that display arrhythmias predominantly during rest or sleep videlicet at slow heart rate. Therefore, the first clinical event of the patient with LQTS3 often is cardiac arrest rather than syncope. About the molecualr mechanism of LQTS, vast majority of mutations in SCN5A produced the disruption of the fast inactivation but not the slow inactivation. The disruption allows the sodium channel reopen and produces the persistent inward current during the action potential plateau phase. Gain of function mutations in sodium channel delays the depolarization of the action potential and causes the prolongation of the action potential.
Brugada syndrome, a familial disease which charactered by ventricular arrhythmia and sudden cardia death even occuring in healthy person at relatively young age (mainly between 30 to 40) and more in male, is first raised by brothers of Brugada in 1992. the features on ECG show the elevation of ST segments in the precordial line. Mutation in SCN5A is acquired as original of the SQTS3 in a familial disorder in 1998. More than 100 mutations in SCN5A is related the Brugada syndrome. Besides the mutation in SCN5A, mutations in the 3-subunits SCN1B and SCN3B, and the regulatory protein GPD1-L have been described in some Brugada syndrome patients. In a word, mutations in SCN5A or ancillary subunit caused to Brugada syndrome becausing of the reduction of sodium channel availabality, loss of function. Some factors cause loss of function in ion channel. For example the decreased trafficking will degrade the number of sodium channel (N) in membrane surface, or disruption of activation, accelerated inactivation, and impaired recovery from inactivation will alter channel gating properties (the open probability and the single channel conductance).
Loss of mutations in SCN5A underlies the mechanism of progressive cardiac conduntance defect (PCCD) due to reduction the sodium channel availability. PCCD is charactered by progressive conduction slowing through the His-Purkinje system, leading to the complete AV block, syncope and sudden death. Same to PCCD, Sick Sinus Syndrome is also caused by the mutations in SCN5A by decreasing the current of inward sodium currents. Sodium channel contribute to the cardiac automaticity owe to the inward sodium currents in depolarizing progress. Therefore, automaticity of sinoatrial pacemake can also be regulated by the sodium channel. Atrial fibrillation is often happened in elderly person with the abnormal heart and younger person with normal structure. In recent, both loss of function and gain of function mutations in SCN5A have been identified as atrial fibrillation due to decrease atrial conductance velocity attributed to the degrade of sodium inward currents and increase atrial action potential duration and excitability owe to the raise of sodium channel avaibality, respectively. In addition, the patients with dilated cardiomyopathy are evoked by the mutations in SCN5A.
In recent years, "overlap syndrome" of cardiac sodium channel deseases have known to exit. The term of "overlap syndrome" is refered to extensive clinical and biophysical overlap. For example, the patients with the mutation SCN5A-1795insD+/- shows extensive variability in type and severity of symptoms of sodium channel disease. Otherwise, the single mutation in SCN5A alone but express pleiotropic effects make further verified through a transgenic mice SCN5A-1798insD+/- (equal to human SCN5A-1795insD+/-). It is further confirmed that a single SCN5A-1795insD+/- mutation is sufficient to express the overlap syndrome of the sodium channel. Heterogenous biophysical properties of ion channel mutations causing the mixed disease expressively are now increasing recognized. Therefore, it is necessary that improve diagnosis for the sodium channelopathies.
Calcium channel
Voltage gated calcium channel is the main channel across by Ca2+ into the intracellular in many excited cell. L-type Cav1.2 channel is an important voltage gated calcium channel and have been detected in many organs. Notwithstanding the widespread important function of the L-type calcium channel, the mutations have been identified is very rare. Mutations of deletion of the pore forming part in Cav1.2 channel change significantly the properties of the channel leading to the embryonically lethal. On the contrary, mutations associated with mold effects on ICa,L kinetics is well adapted and the patients with no obvious symptoms.
Structure of L-type CaV1.2 channel
Similar to the voltage-gated sodium channel, structure of L-type CaV1.2 channel is formed by a pore-forming subunit divided for four dormains (from I to II). Each domain consists of six transmembrane segments (S1 to S6). As the common to other voltage gated channel, the S4 of each domain is the voltage sensor moving across the membrane corresponding to the depolarization of the membrane potential. The part of S5, S6 and the linker S5-6 in each domain are composed to the ion conduction pathway.
Fig. 2. Location of the mutations identified in CaV1.2 pore-forming a 1 subunit. Red circles (G402S and G406R) reprents spliced cardiac exon. Blue circle (G406R) is located in smooth muscle exon. Purple circles (A39V and G490R) represents constitutive exons. b Computer modeling revealed cardiac action potential when the mutation in smooth muscle exon and in cardiac exon.
Ancillary subunits
To date, four 3 subunits of calcium channel genes are expressed in the heart. The ancillary subunits can in theory bind to the Cav1.2 subunit at the a1 interaction domain (AID). The domain is highly conserved binding motif of 18 amino acid residues present in the cytoplasmic linker between repeat I and II of a1 subunits. The 32 subunit is generally believed to constitute the intracellular, accessory subunit of the Cav1.2 channel in adult mammalian myocardium.
There are two distinct function of P subunit binding with the pore-forming subunit: before binding as a chaperone helping a subunit correct location at the membrane, after binding as an allosteric modulator to regulate the kinetic of the currents. Otherwise, different P subunit increase the currents of ICa,L at different levels by increasing the the channel opening probability, produce distinctive effects on channel inactivation kinetics and induce hyperpolarizing shifts in the voltage-dependence of channel activation.
Recent studies have shown that the ancillary subunits are members of the membrane-associated guanylate kinase (MAGUK) family of proteins by crystallographic information. Therefore, the ancillary subunits, as a ideal targets, interact with other protein such as ahnak or various members of the Gem/kir family of small Ras-like GTPase.
Functions of L-type Cav1.2 in heart
The L-type Cav1.2 channel plays a critical and dominant role in triggering excitation-contraction coupling in cardiomycytes through the influx of the calcium ions to form the plateau of the ventricular action potential. Otherwise, the L-type Cav1.2 channel contribute to the trigger of the contraction when initiate the Ca2+ from the sarcoplasmic reticulum.
LQTS8 (also called Timothy syndrome) is first reported in 1990s with the symptoms of syndactyly. The molecular basis of Timothy Sydrome is the mutations in L-type Cav1.2 at IS6 segment encoded by the two mutually exclusive exons 8/8a. Timothy syndrome is several features. Firstly, mutations are common caused by the deamination of a methylated cytosine to a thymine at de nove. Secondly, gain of function of L-type Cav1.2 lead to the increase of current density of ICa,L through slowing the inactivation of the channel. The net effects of the mutations increase of intracellular calcium ion. Thirdly, mutations are common in the mutually exclusive exons. The mutation of exons alters dramatically channel properties and the unaffected exon function normally. Lastly, mutation in Cav1.2 is also related to normal function of immune system.
Different Cav1.2.variants are formed by the extensive alternative splicing by changing the pattern of splicing causing a series of disease. Among of them, only a few major splicing site can be divided to the cardiac and smooth muscle subfamily. In human heart, Cav1.2 channel contained cardiac exon may be 77 percent of all Cav1.2 channel. The first Timothy Syndrome mutation G406R is at the smooth muscle exon whereas other mutation G406R and F402s is at the cardiac exon. The latter can be named as TS2 and the cardiac arrhythmia of what is more serous than TS.
TS2 mutant Cav1.2 belong to gain of function mutation leading to more calcium ion into the cytoplasm. Therefore, the action potential duration are be prolonged and a longer QT interval on ECG is shown. Comparision with TS (mutaions at smooth muscle exon), TS2 (mutations at cardiac exon) produces more effects on action potential duration and excitability. For example, the prolongation of action potential duration by mutation at cardiac exon and smooth muscle exon is 30% and 8% by computer analysis, respectively. Therefore, symptom of patient with TS is molder than one with TS2.
Otherwise, loss of function mutation in Cav1.2 or ancillary subunit cause to Brugada syndrome charactered by the elevating QT segments and shortening QT interval on ECG. The ages of the patients with Brugada syndrome is ranging from 21 to 44 years old which is elder than TS patients. Besides the dysfunction in cardiac tissues, other organs are nearly normal in patient with Brugada syndrome.
Potassium channel
Potassium channel is the largest family of ion channel protein and divided into voltage- and ligand- potassium channel owing to activating by voltage and ligand, respectively. Most of potassium channel are determined and depended by membrane potential. The ion-conducting pore of a K+ channel is formed by four a-subunits that co-assemble as homo- or hetero-tetramers with different biophysical properties. Their gating characteristics can also be modulated by ancillary subunits or all kinds of blocker or activator.
Classification of the cardiac potassium channel
On the basis of their function, cardiac K+ channels are further classified into the transient outward channels, the delayed rectifier channels and the inward rectifier channels (Figure 1). Firstly, the transient outward current (Ito) formed by Kv4.3 manifests rapid activation and subsequent inactivation during the early repolarization phase (at phase 1). (ii) The delayed rectifier channels consist of at least three members Kv1.5, Kv11.1 and Kv7.1, for three different currents IKur, IKr, and IKs, respectively. All three channels activate at positive potentials but with distinct time courses, for example ultrarapid, rapid, and slow, respectively. Inactivation of IKur and IKs is slow, on the contrary that of IKr is extremely fast. (iii) The cardiac inward rectifier potassium channels have more than three components. The major classical of one is Kir2,1-2.3 which form IK1 currents. This channel is always open and conducts K+ better into than out of the cell. Another channel expressed in atrial myocytes is an acetylcholine-dependent channel whicn conducts IK,Ach corresponding with the stimulation of G-protein-coupled muscarinic (M2) and adenosine (A1) receptors. The activation of IK,ACh shortens the active potential duration (APD). The third one in cardiomyocytes is ordinary closed under physiological metabolic conditions and is activated when the cells are deprived of intra-cellular adenosine triphosphate (ATP). Similar to IK,ACh, IK,ATP causes profound APD shortening.
Notwithstanding general similarity in the mechanism of action potential arised, the distribution of potassium channel in cardiac ventricular myocardium and cardiac atrium is the most striking difference. For example, IKur and IK,Ach currents are both detected just in atrial but not in ventricular. Under the positive potential IKur is activated rapidly followed by ICa,L activation and therefore lead to less positive plateau phase in atrial than ventricular cells.
Structure of potassium channel
Remarkable advances about the structure-function relationship in ion channel have great progress in over past 30 years. Especially the progress in two experimental techniques, one is the single channel conductance recorded limpidly and plainly by patch clamp and another is the first determination at atomic-resolution of the structure of potassium channel protein. By means of these techniques, we can monitor real-time behaviour of single macromolecule in cell membrane and associate the behaviour with the molecular architecture of the protein
Structures of voltage gated potassium channel
Voltage-gated potassium channel is a homotetramer formed by each subunit containing six transmembrane domains (S1-S6). The pore domain comprise of the S5, the pore helix and S6 segment. The S1-S4 segments of each subunit form the voltage sensor domains (VSD). The part of VCD regulates the open and close of the pore domain through moving across membrane in response to change of membrane potential. The channel pore is anisomerous and its dimensions change when the transition of channel gates from a closed to an open state. The K+-selectivity filter, a narrow cylinder, exists in the extracellular end of the pore that optimally utilize for conduction of K+ ions. The difference of the selectivity filter of Kv channels with other channel is charactered by the highly conserved sequence Thr-Val-Gly-Tyr-Gly (the K+ signature sequence), located at the carboxy-terminal end of the pore helix. In hERG channel, the Thr and Tyr residues are substituted with Ser and Phe. The hydroxyl group of Thr in side-chain and the carbonyl oxygen atoms of the other four residues in each subunit all expose to the narrow K+ selectivity filter. These atoms mentioned above (OH- and O) encircle several octahedral binding sites that compete with the single water molecule of hydrated K+ ions and make a single water molecule alone arranged in a single line pass across the filter.
The central cavity under the selectivity filter is much more widen and is a filled water region boundary by the S6 a-helices. In the closed state, the four S6 domains criss-cross near the cytoplasmic interface to form a narrow aperture that is too small to permit entry of ions from the cytoplasm. In response to membrane depolarization, the S6 a-helices splay outwards and increase the diameter of the aperture to allow passage of ions.
Structures of Kir channel
Inward rectifier K+ channels (Kirs) consist of two transmembrane domains (M1 and M2). Ml and M2, equal to the S5 and S6 part of voltage-gated potassium channel, is connected by a pore containing the G(Y/F)G sequence. In addition, the Kirs channels comprise of intracellular N- and C-termini. This architecture is typical structure of KATP and Kirchannels. They conduct K+ currents more in the inward direction than the outward and play an important role in setting the resting potential close to the equilibrium potential for K+ (EK, approximately -90 mV for [K+]o = 5 mM) and in repolarization. Kir channels form either homo- or heterotetramers.
About the essential properties of rectification which attributed to blocking of Kir2 channels by intracellular organic cations called polyamines response to potent and strongly voltage-dependent. Of the polyamines, free spermine in cell is the most potent inducer of inward rectification, followed by spermidine, putrescine, and then Mg2+. Accordingly, the "activation" of inward rectifiers upon membrane hyperpolarization is essentially uncoupling of polyamines or Mg2+ from the Kir channel pore.
The general architecture of the Kir channels and the key structures involved in permeation and block is well established. Similar to bacterial homologs, the Kir channel in mammalian has a selective filter at the extracellular of the membrane with a signature sequence GYG. Under the filter there are a widen water cavity towards the intracellular of the membrane. There are a number of residues in Kir2 critical for inward rectification. For example, Mutations of D172 located at the level of the water cavity is firstly identified, a ‘rectification controller’. Spermine has high affinity with D172 in the vincity of the filter and unbinding from the residues highly voltage-dependent. Another important residue in rectification is E224 and E299 in the cytoplasmic region which form a ring of acidic. Contrast to D172, spermine has a low-affinity binding with E224 and E299 and low voltage-dependent. Spermine, as the largest (~16-18 A) polyamine, the pore of the Kir2 is long enough to easily accommodate two or more spermine.
In native IK,ACh channels, spermine can also induce strong inward rectification. There are half of the residues in underlying Kir3.1/Kir3.4 channels equal to D172 and E224 in Kir2.1. The negative residues in Kir3.1/Kir3.4 channels have important role causing strong inward rectification. Although Kir2 and Kir3 have many common similarities there are lots of differences in the kinetical properties between both of them (Anumonwo and Lopatin, 2011).
Kv11.1
Kvll.l formed a kind of the delayed rectifier currents IKr encoded by KCNH2 which is identified as the molecular basis of LQT2 in 1995. To date, nearly 300 different mutations of Kvll.l is the direct reason of congenital LQTs (http://www.fsm.it/cardmoc/; see Table 1) and almost all drugs induced acquired LQTS do so through interaction with the hERG channel. Besides that, dysfunction of Kv11.1 may cause short QT syndrome and artrial fibrillation. Therefore, Kv11.1 has vital role in excitability and action potential conductance in heart
The features of the structure
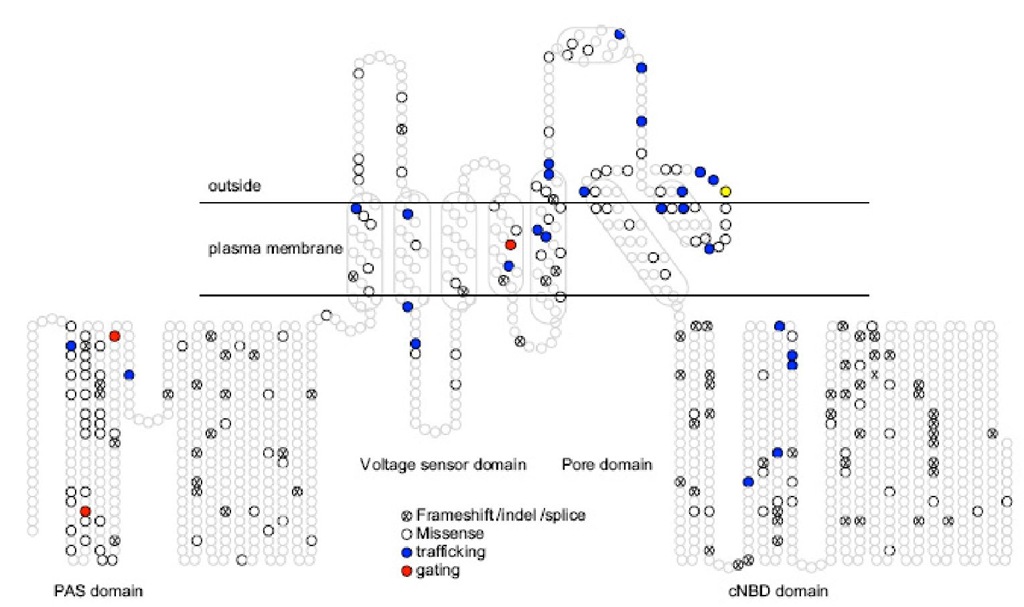
Differences from other Kv potassium channel, hERG channel has a unique extracellular part between S5 and the "pore", so called "S5P linker" that contained an amphipathic helix. With exception of transmembrane segment, hERG has intracellular N-terminal and C-terminal. The N-terminal has a Per-Arnt-Sim (PAS) domain which is unique to hERG channe in mammalian ion channel and play a role in deactivation of the channel. The C-terminal has a cyclic nucleotide binding domain (CNBD) which has relatively little effect on gating by binding with cAMP. However, mutation of the domain cause trafficking defects followed by loss of function of hERG channel and the last lead to cardiac arrhythmia.
Kinetic characters of Kv11.1
Similar to other Kv potassium channel, hERG channel exits at least three distinct conformational states: closed, open and inactivated. Transition from closed to open states or from open to inactivated state of channel attributed to the activation or inactivation which evokes the constrain of the conduction pathway and disrupted ion translocation. hERG channel have significant homology to other Kv family members by sequence analysis. However, kinetics characters of hERG channel activation and inactivation is distinct with other Kv channel. Contrast with other Kv channel, activation of hERG channel is much slower (ion ranging from 100s of ms to many second) and inactivation is more rapid (ioff ranging from 1 to 10 ms) and voltage-dependent. Because of the slow activation of hERG at depolarized potentials, little outward currents produced by the channel flows through the phase 1 and 2 of cardiac action potential. Reduced outward currents conduce to the maintenance of the plateau of cardiac action potential by allowing Ca2+ entry and avoid the cell refractory to premature excitation. In addition, the increase outward currents, due to the much faster recovering from inactivation, is the most important determination of the plateau of cardiac action potential. Besides that the distinct gating kinetics of hERG channel leads to form the character IKur currents which is help for suppression of propagation of premature beats. Therefore, hERG channel has crucial role in normal or abnormal cardiac action potential.
Activation
The gate of potassium channel is the bundle crossing formed by four the intracellular parts of S6 transmembrane helices of each subunit. The gate at closed state is too narrow to allow transverse of K+ ions. Transition to open state attribute to these helices which kink at a gate hinge revoking to enlargement of the pore and allow potassium ion pass it. In the bacterial KcsA, MthK and KvAP channels, a conserved Gly residue in S6 is proposed to serve as the hinge for the activation gate. Mutation of the putative Gly hinge in hERG alters gating but does not prevent channel opening. Although Kv1-Kv4 channels also have a Gly in the same location, a different molecular hinge may mediate channel activation. Therefore, the gating hinge, common formed by PVP motif in Kv1-Kv4, has vital roles in change of channel gate whereas the second proline of PVP motif was replaced by glycine in hERG channel.
The S1-S4 VSDs also have important role in regulating the transition from closed to open state in voltage-gated potassium channel. The voltage sensor in hERG channel which is the six basic positive amino acid every 3 residues localized in the position between 525 and 538 of the S4 domain Especially, the most important amino acid is the K525, R528 and K538 conducing to voltage sensing for slow activation. With exception of the positive residues, the acidic amino acid in S1-S4 stablize the VSD at open and closed conformation through forming salt bridge with S4 residues.
It is well known that the voltage sensing regulating the channel open or closed by voltage sensor domain (VSDs) up or down across the transmembranes. However, to date the exact rearrangement of the structure between up or down and the exact magnitude of movement of VSD is still debated. For instance, although the structure of mammalian Kv1.2 channel at open state, especially the location of VSD up relative to the membrane have revealed. But crystal structure of the channel at closed state scilicet the down relative to the membrane do not still detected. Another meaning thing is about the distinct kinetics of the hERG channel because the overall of SVD is high homology with other members of Kv channel family by analysis sequence and hydropathy plots.
To find the reason that the kinetics of hERG is so different, many scientists are attracted in the field and find several key pieces of evidence. When the gating currents corresponding to the movement of voltage sensor domain are measured at the same time the results show that a slow time course corresponding with the slow the activation. From the results we can conclude that the slow activation of hERG attribute to the slow movements of VSDs
Inactivation
About the hERG channel, the mechanism of the inactivation is the C-type at original stage. However, hERG inactivation is orders of magnitude faster than C-type inactivation and its intrinsically voltage-dependent. Many papers pay attention to the molecular basis of the voltage-sensitive of channel inactivation and the relationship between activation and inactivation gating, whether the process are couple or completely separate. Some data indicate that other part but not the S4 contribute to regulate the hERG inactivation. Ser620 and Ser 631 in the P-domain are vital for inactivation. In addition, the charge change of S5P can markedly alter the inactivation of hERG. Therefore, Perrin conclude that different parts of voltage sensor domain participate in regulate the channel activation and inactivation. The amphipathic a-helix of S5P contain in the regulation of hERG inactivaon, due to the relative movements between the a-helix of S5P and the pore domain.
The regulation of KCNE2
It is well known that KCNE2 (Mirp1) was described as a modulator of the ether-a-go-go-related gene 1 (ERG1) potassium current. The protein of KCNE2 is a single transmembrane peptide with an intracellular C-terminal and an extracellular N-terminal. Coexpression with Kv11.1 increase the currents of IKr owing to increasing the single conductance, altering the kinetic characters of inactivation and inactivation. Later KCNE2 was found to also change the KCNQ1 potassium current by drastically changing the gating properties. Mutations in KCNE2 are associated with long QT syndrome (LQT6) (http://www.fsm.it/cardmoc/) because of a decreasing influence on both ERG1 and KCNQ1 currents by KCNE2 mutation. Accordingly, both types of complexes KCNQ1/KCNE1 and KCNH2/KCNE2 could play a functional role in the heart.
Functions of Kv11.1 in heart
Loss of function and gain of function mutations in Kv11.1 produce to the formation and conductance of action potential in cardiac tissues. Loss of function mutations decreases the currents of IKr due to decrease of channel number, channel open probability and single channel conductance as mentioned above.
Fig. 3.Topological map of position of nearly 300 different mutations in hERG in LQTS2.