Abnormal Breathing Patterns and Sleep Reports
Brain injury and certain drugs and toxins affect breathing patterns during both wakefulness and sleep; however, there is a growing awareness of how abnormal breathing only during sleep may affect health. Periods of cessation of airflow into and out of the lungs (apnea) regularly occur at sleep onset, and episodes of partial upper airway obstruction during inspiration (snoring) are very common. Some irregularity of breathing is considered normal during sleep, including mild CO2 retention and a reduction in PaO2, as well as irregular breathing at sleep onset or with dreaming. As with many biologic phenomena, breathing irregularities that occur during sleep are designated as abnormalities only if they are sufficient in magnitude and frequency to disrupt sleep continuity or impair oxygenation enough to affect a person during wakefulness.
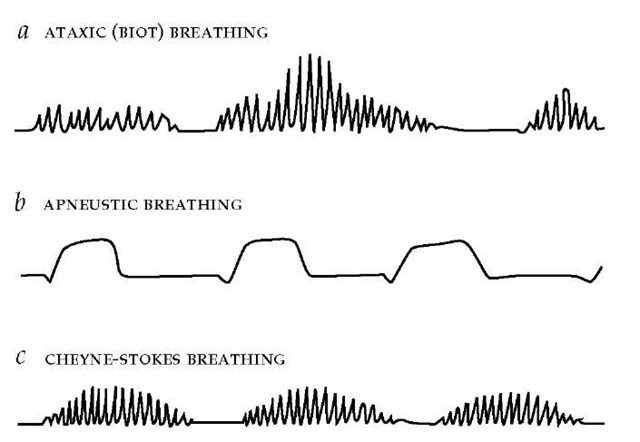
Ataxic and apneustic breathing
Ataxic (Biot) breathing is a random pattern of shallow and deep breaths interspersed with irregular pauses [see Figure 3]. Ataxic breathing results from disruption of medullary neural pathways by trauma, hemorrhage, or extrinsic compression caused by cerebellar or pontine hemorrhage; it can be seen in terminally ill patients because respiratory control systems are affected by multisystem failure.12 Complete apnea may ensue, especially in patients given sedative or narcotic drugs. Another disturbance, apneustic breathing, is characterized by an end-inspiratory pause of 2 to 3 seconds before exhalation is begun [see Figure 3]. Ap-neustic breathing is associated with caudal pontine lesions and is sometimes intermixed with ataxic breathing patterns.
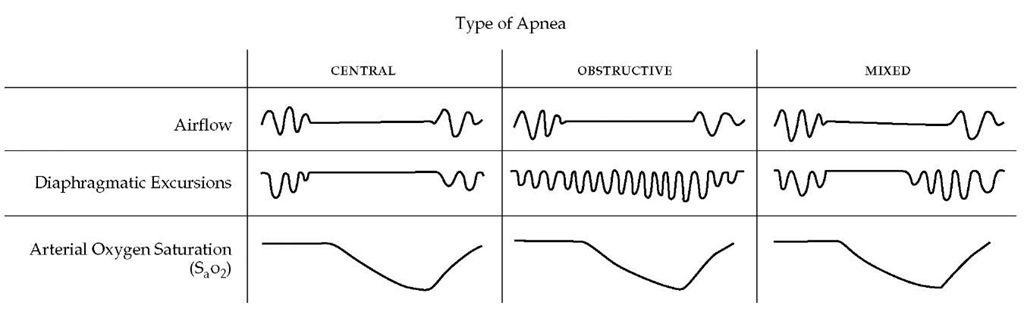
Three patterns of apnea, or cessation of breathing, can be observed during sleep. These apneas are defined as episodes of a reduction in airflow of more than 80% occurring for more than 10 seconds.13 Apneas may be classified as central (or nonobstructive), obstructive, or mixed [see Figure 4]. In central apnea, which implies a cessation of respiratory activity at a brain stem level, both airflow and respiratory efforts are absent. During obstructive apnea, respiratory efforts persist, although airflow is absent at the nose and mouth. Obstructive and central apneas are related clinically and pathophysiologically. Many adult patients exhibit mixed apneas in which both central and obstructive patterns occur. In a single apneic episode, there may be a period in which no efforts occur, followed by the appearance of respiratory efforts, also without airflow. In addition, in the same night, patients may have all three types of apneas in varying proportion. If more than 80% of apneas are of a central type, the patient is classified as having central sleep apnea. If apneas are predominantly mixed and obstructive apneas, the patient is classified as having obstructive apnea.
Figure 3 Irregular breathing patterns may reflect central nervous system disease or an inherent alteration in the apneic threshold. Three examples of irregular breathing are illustrated: (a) Ataxic breathing is characterized by an unpredictable sequence of breaths varying in rate and depth and is associated with medullary disease. (b) Apneustic breathing involves repetitive gasps, with pauses at full inspiration lasting a few seconds, and is associated with pontine disease. (c) Cheyne-Stokes respiration is cyclic, with a crescendo-decrescendo pattern interrupted by apneas.
Hypopneas or hypoventilation during sleep may arise by mechanisms similar to those producing apnea. Hypopneas are defined as episodes of a reduction in airflow of 30% to 80% occurring for more that 10 seconds.13 Hypoventilation (hypopnea) leads to increased CO2 and decreased O2 levels in arterial blood and causes arousals from sleep; as with apneas, hypopnea may result from reduction in respiratory efforts or partial upper airway obstruction. Snoring is a form of partial airway obstruction and is called obstructive hypopnea. Snoring is common, but some patients who snore have symptoms similar to those of sleep apnea syndrome even if complete cessation of airflow (ap-nea) never occurs during sleep. Moreover, such patients may exhibit abnormal sleep and cardiorespiratory changes.
Ventilatory behavior in sleep
The transition from wakefulness to non-rapid eye movement (NREM) sleep is accompanied by a reduction in metabolic rate and therefore a reduced need to breathe. Consequences of sleep onset include reduced tidal volume, changes in lung mechanics, reduced activity and upper airway dilators, reduced upper airway caliber, and loss of load compensation [see Load Compensation, below].
Sleep is accompanied by reduced postural muscle tone. In NREM sleep, the ratio of rib cage displacement to abdominal displacement is greater than it is during wakefulness, whereas in REM sleep it is less.15 These changes in displacement may affect the distribution of ventilation in the lungs, increasing ventilation-perfusion mismatching and contributing to hypoxia; the development of hypoxia, in turn, may necessitate changes in respiratory output, which may initiate an unstable breathing pattern.
Upper Airway Function
Upper airway caliber is reduced during sleep, and air passage is further impaired by decreased activity of upper airway mus-cles,16,17 especially the muscles involved with tonic activity (independent of the phase of respiration), such as the tensor veli pala-tini muscle.18 The mechanical consequence of reduced airway caliber is increased upper airway resistance.19 Because pharyn-geal compliance increases during NREM sleep, negative in-trathoracic pressures normally produced in the upper airway during inspiration will result in airway collapse. Even in healthy persons, negative intrathoracic pressure during NREM sleep limits inspiratory flow, resulting in an inspiratory plateau that persists in the presence of increasing negative pressure.
Curiously, the retropalatal airway is less compliant during REM sleep, when muscle activity is much reduced, than during NREM sleep.20 This finding points to the significance of nonneu-romuscular factors (e.g., bony and cartilaginous support) in the maintenance of upper airway patency during sleep.
Load Compensation
When the ratio of load to inhalation is increased (whether because of resistive factors or obstructive factors), a concomitant increase in breathing effort is required to restore tidal volume (i.e., load compensation). During sleep, however, immediate and subsequent load compensation is compromised and results in decreased tidal volume and minute ventilation, which thereby results in alveolar hypoventilation. The ensuing elevation of arterial PaCO2 restores CO2 elimination toward normal levels.7 The inability to perceive and immediately respond to increased loads allows for sleep to continue undisturbed. Thus, the main consequence of sleep is an increase in PaCO2 of 4 to 5 mm Hg. Such elevations in PaCO2 result in mild acidosis in both healthy persons and in persons with cardiopulmonary disorders but without sleep-disordered breathing (SDB).
Heavy snorers may not arouse from sleep despite continuous generation of subatmospheric intraluminal pressure that is several times higher than that which occurs during wakefulness [see Figure 5]. If increased resistance and inspiratory flow limitation are prolonged, the increased work of breathing or hypoventila-tion, or both, leads to respiratory-related arousals (RERA) from sleep. Partial obstruction of the upper airway (with RERAs) and daytime sleepiness are the features associated with upper airway resistance syndrome (UARS).21
The Hypocapnic-Apneic Threshold
In NREM sleep, a highly reproducible hypocapnic-apneic threshold is unmasked, and a central apnea will occur if the PaCO2 is lowered, even by a small amount.22 As a result, hypocapnia is the most important inhibitory factor to breathing during NREM sleep. This threshold level of PaCO2 is decreased by hypoxia, possibly by excitation caused by miscellaneous nonchemical stimuli. One major cause of SDB is breathing instability produced by this threshold effect and by arousals, hypoxia, and other factors that alter this threshold over time.
Sleep Effects on Cardiovascular Physiology
The cardiovascular system adjusts to the changes in gas exchange that accompany sleep and to the apneas and hypopneas that may interrupt sleep. Normally, during NREM sleep there is a withdrawal of sympathetic tone, both neural and humoral, and an increase in parasympathetic tone—changes that result in a reduction in heart rate, blood pressure, and cardiac output.23 The decreased cardiac workload and O2 demand are accompanied by a diminished ability to provoke an arrhythmia.
Gradual awakening is accompanied by a modest increase in sympathetic outflow without much evidence of parasympathet-ic withdrawal. In contrast, with an abrupt arousal caused by noise or sleep apnea, there occurs an abrupt increase in sympathetic drive manifested by increases in blood pressure and heart rate and by marked parasympathetic withdrawal.
In REM sleep, cardiovascular and breathing systems are relatively independent of metabolic drive and inhibition of muscle activity. Sympathetic activation increases to levels seen during wakefulness but is often episodic, leading to transient changes in heart rate, blood pressure, and breathing. Such surges in blood pressure may play a part in triggering ischemic events in patients with heart disease or diabetes.
In general, however, sleep is cardioprotective in healthy persons. Sleep apnea disrupts cardiovascular regulation during sleep because of repetitive arousals, hypoxemia, and increased intrathoracic pressure changes, which result in preload and af-terload effects on the heart.
Figure 4 This schematic representation of the ventilatory signals recorded during a sleep study (polysomnogram) illustrates the different patterns found in central, obstructive, and mixed apneas. In each example, the presence of apnea is confirmed by the cessation of airflow at the nose and mouth (top), and the consequence of apnea—namely, hypoxemia—is demonstrated by the development of oxygen desaturation on the continuous record of arterial oxygen saturation (SaO2) (bottom). The three types of apnea are distinguished by the respiratory efforts made during the episode (middle). In central apnea, no respiratory efforts are made; in obstructive apnea, diaphragmatic contractions continue and often intensify during the episode; and in mixed apnea, a period of absent respiratory efforts is followed by active inspiratory muscle contractions against an occluded upper airway.
Figure 5 Several cycles of breathing during EEG-defined wakefulness and sleep are shown for wakefulness (left) and quiet sleep (right) in a healthy person. Flow limitation is present during inspiration, with a chopped-off flow pattern and rapid fluttering of flow indicative of snoring. This pattern can represent a steady-state condition, balancing load compensation and chemical drive.
Evaluating sleep disturbances
Monitoring a person with electrodes during sleep results in a classification of sleep into two states: non-rapid eye movement, or NREM, sleep and rapid eye movement, or REM, sleep. NREM sleep can be further subdivided into stages I and II (light or transitional sleep) and stages III and IV (deep sleep), depending on the frequency and amplitude of brain waves. States are distinguished by recording electroencephalogram (EEG), electro-oculogram (EOG), and electromyogram (EMG) measurements. The combination of these measures and the cardiopulmonary monitoring of airflow, respiratory effort, oxygen saturation, and heart rate—along with identification of body position—constitute polysomnography, which is a common test used to diagnose sleep apnea.
Respiratory Disturbance
Various measurements are used to quantify respiratory disturbances during sleep.15 The apnea-hypopnea index (AHI) is the total number of apneas and hypopneas occurring during sleep divided by the hours of sleep time. Values of AHI can be computed for the different stages of sleep. Another term for AHI is the respiratory disturbance index (RDI). The term desaturation index, also called oxygen desaturation index, refers to the number of times per hour that O2 saturation falls by more than 3% to 4%, and it may be reported as an independent measure of car-diorespiratory instability. The snoring index (SI) is the percentage of time spent snoring during sleep.
The arousal index (AI) is computed as the number of transient awakenings per hour, and it is defined by a change in state from sleep to waking that is longer than 2 seconds but less than 3 minutes.13 This number is used to estimate individual exposure to transient arousals from sleep, and it is distinguished from nocturnal awakenings by the length of the bout of wakefulness. Included in this index are spontaneous brief awakenings caused by external and internal stimuli (e.g., noises and leg jerks, respectively). The AI may differ from the AHI or RDI because many (approximately 20%) apneas or hypopneas are not accompanied by arousals, and because the AI count includes arousals that are not apnea-induced.
Oxygen Saturation
Various measurements of O2 saturation, as plotted over time, indicate the extent of exposure to hypoxemia during sleep. One measure is the O2 saturation profile, in which values of O2 saturation are presented in the frequency domain, which plots the pattern and extent of O2 deficiency during sleep.26 Values reported include estimations of the lowest O2 saturation and the length of time spent below a specific O2 saturation (e.g., 90%, 85%, 80% O2 saturation of hemoglobin). In addition, recordings can be examined in the time domain to estimate the degree to which O2 saturation exhibits periodic behavior.
Hypoventilation
Hypoventilation is not directly measured during routine sleep studies, because tests for arterial blood gases are uncomfortable and incur an unfavorable risk-to-benefit ratio. Markers for PaCO2 include end-tidal values of CO2 or transcutaneous estimates of CO2. Both are qualitative. The former makes the assumption of adequate sampling of alveolar gas, and the latter provides trends rather than precise numbers. Neither is used routinely during sleep studies in adults.
Sleep-Disordered Breathing
Healthy individuals may exhibit obstructive or central apneas at sleep onset or during periods of REM sleep.27 Episodes are usually less than 15 seconds in duration and are not repetitive.
Occasionally, longer periods of apnea (lasting 30 seconds or more) are seen during REM sleep. These episodes may not be accompanied by arousal or sleep-state changes.
Healthy young men have more frequent apneas during sleep than young women, but after the sixth decade of life, respiratory disturbances during sleep increase in number and occur with equal frequency in men and women.27 Patients with a clinically important sleep apnea may be distinguished from patients with normal respiratory disturbances by the presence of repetitive ap-neas longer than 10 to 15 seconds that occur during stages I and II of NREM sleep and during REM sleep and that are frequently accompanied by daytime sleepiness. If treated, patients with significant apnea show improvement in daytime symptoms and general performance.
Definition of sleep-disordered breathing
In the United States, 9% to 12% of women and 27% to 35% of men may have an AHI greater than 5, a number often quoted as a threshold value for normality; however, many people with an AHI greater than 5 have no clinically apparent illness.27 If the definition of illness is the presence of daytime sleepiness or cardiovascular complications such as hypertension, it is estimated that about 2% of women and about 4% of men have symptomatic SDB. Studies suggest that patients with symptomatic SDB who drive are at increased risk for vehicular accidents in which they may incur substantial disability. Medical practitioners often fail to recognize the presence of sleep apnea syndrome.
Etiology of sleep-disordered breathing
Predisposing Factors
Snoring is generally considered a predisposing feature in the development of SDB and symptoms of sleep apnea.28 Snoring increases with age; approximately 45% of men and 30% of women 65 years of age or older are said to snore. Persons who snore are two to three times more likely to have hypertension29 and 1.5 times more likely to have diabetes than people who do not snore, even after age and obesity are taken into account as risk factors for these diseases.30
Genetic Factors
Sleep apnea has a genetic component. Symptoms relating to apnea occur two to four times more often in family members of affected patients than in a control population. Sleep ap-nea events occur more often in first-degree relatives of sleep apnea patients than in control subjects matched for age, sex, and socioeconomic status. Such studies reveal that the symptomatic sequelae of multiple apneas are quite variable, probably because of an interaction between both genetics and the environment.31
Patients with sleep apnea exhibit a twofold increase in a polymorphism for apolipoprotein E associated with cardiovascular disease and Alzheimer disease.32 Using a statistical approach to estimate inheritance, the Cleveland Family Study found that 27% of the variation in AHI in the community could be accounted for by perhaps only a few genetic factors.33 Transmission patterns in both the white and the African-American patients were consistent with mendelian inheritance. Adjustment for body mass index (BMI) significantly reduced the significance of a genetic effect in whites but not in African Americans. Thus, an underlying genetic basis for sleep apnea could be independent of the contribution of BMI to the disease in African Americans.
Specific craniofacial morphology (e.g., a short mandible and round head) are predisposing factors for the development of snoring, apneas, or both.34 It is also known that there are familial traits in hypercapnic and hypoxic sensitivity; these could relate to the tendency to breathe periodically during sleep.35 In addition, obesity and alcoholism (factors associated with SDB) can be family traits and, to the extent that these factors are causally related to apneas, may account for the familial clustering of sleep apnea. It is not known whether there is a familial trait involving the respiratory coordination of muscles of the chest wall and upper airway. A role for genetic transmission of ventilatory behavior (respiratory frequency, tidal volume, and minute ventilation) is directly supported by reports of nearly absent respiratory depression in several gene knockout models and by studies of inbred rat and mouse strains.36 Given the current evidence, sleep apnea does not appear to be the result of a single mutation or protein action.