Valvular heart disease is an important cause of cardiac morbidity in developed countries despite a decline in the prevalence of rheumatic disease in those countries. Valvular heart disease can give rise to stenosis, regurgitation, or a combination of lesions at one or more valves. The more common significant anomalies currently encountered are mitral regurgitation, caused by mitral valve prolapse; aortic stenosis, caused by a congenital bicuspid valve or by senile valvular calcification; and aortic regurgitation, caused by a bicuspid aortic valve or dilatation of the aorta. Valvular lesions can occur as a result of pathologic changes in the valve leaflets or supporting structures (i.e., the chordae or papillary muscles). Ventricular or aortic enlargement can also produce valvular regurgitation as a result of annular dilatation and inadequate leaflet coaptation in the absence of any specific valve pathology. Valvular heart disease tends to progress over time as degenerative changes are superimposed on the primary pathology. Iatrogenic causes of valvular disease are increasingly recognized. Common causes of major valvular lesions are listed [see Table I].1
Etiology
Congenital disorders
Anomalies in the development of valve cusps are common at the aortic or pulmonary positions. The normal configuration of these valves is tricuspid. Stenosis is the rule when only one cusp develops, whereas bicuspid valves may be stenotic or regurgitant [see Figure I]. Congenital anomalies of the atrioventricular valves are uncommon; the most common abnormality is congenital cleft mitral valve. Valvular abnormalities can be seen in specific developmental syndromes, such as pulmonary stenosis in rubella syndrome and supravalvular aortic stenosis in Williams syndrome.
Myxomatous degeneration
Myxomatous degeneration most often involves the mitral or tricuspid valve. In this condition, leaflet tissue, particularly chor-dal tissue, is abnormally extensible and weak. The affected valves are therefore more likely to prolapse, leading to significant regurgitation. Chordal rupture is common and may precipitate a rapid clinical deterioration from sudden severe regurgitation. The precise abnormality in valvular tissue is unknown but is thought to involve the structural proteins, such as collagen.2 A familial tendency is often noted in this disease.3 Inherited connective tissue diseases such as Marfan syndrome produce valvular abnormalities similar to those found in myxomatous degeneration.
Rheumatic heart disease
Rheumatic heart disease remains the most common cause of mitral stenosis and a frequent cause of aortic regurgitation. It is the most common cause of multivalvular heart disease. Isolated outbreaks of rheumatic fever continue to be reported in the United States, even in affluent communities.4 Rheumatic heart disease remains a significant problem in immigrants, especially those from Latin America and Southeast Asia. Rheumatic fever appears to cause valvular heart disease by an autoimmune phenomenon whereby antibodies against streptococcal antigens cross-react with valvular tissue. Valvular involvement can present acutely as a result of edema of valvular tissue. Progressive fibrosis, superimposed calcification, and scarring with retraction of leaflet tissue lead to valvular stenosis, incompetence, or both. The interval between the occurrence of rheumatic fever and clinical manifestations varies, as does the degree of involvement. Both mitral and aortic valves are usually involved.
Degenerative disease
Degenerative calcification is a cause of aortic stenosis in the elderly and in patients with renal dysfunction; it results from calcium deposition on the body of the valvular leaflets rather than on the commissures [see Figure I]. Factors found to promote degenerative valvular changes are increasing age, a low body mass index, hypertension, and hyperlipidemia. Histologic changes that simulate atheroma and involve lipid deposition and inflammatory cell infiltration of the leaflets have been described in patients with early degenerative changes in the aortic leaflets. Even mild degenerative changes in the aortic valve have been reported to be adverse prognostic factors.5 Calcification of the mitral annulus is common in the elderly; it is more common in women than in men and can produce mitral regurgitation. Occasionally, mitral annular calcification extends onto the valvular leaflets, causing stenosis.
Endocarditis
Endocarditis usually occurs on previously abnormal valves, although overwhelming sepsis can infect normal valves. The predominant hemodynamic manifestation of endocarditis is valvular regurgitation. Contributory causes of endocarditis include leaflet prolapse (resulting from a large vegetation), leaflet perforation, and chronic scarring of infected tissue. In rare cases, large vegetations lead to valvular stenosis.
Coronary artery disease
Mitral regurgitation is common in coronary artery disease; it has a number of causal mechanisms. Acute ischemia or infarction of a papillary muscle or of the wall to which the papillary muscle is attached leads to impaired leaflet coaptation and mitral regurgitation. Regurgitation can be severe and can vary with the severity of the ischemia. Papillary head rupture or, more rarely, muscle rupture, leads to catastrophic regurgitation that is often fatal.
Table I Causes of Specific Valvular Lesions
|
Mitral |
Aortic |
Tricuspid |
Pulmonary |
|
|
Stenosis |
Rheumatic disease, calcification, SLE |
Calcification, congenital disease, rheumatic disease |
Rheumatic disease, carcinoid tumor |
Congenital disease, carcinoid tumor |
|
Regurgitation |
Myxomatous degeneration, ischemia, secondary causes, rheumatic disease, annular calcification, endocarditis, SLE |
Congenital disease, secondary causes, rheumatic disease, endocarditis, SLE |
Secondary causes, rheumatic disease, endocarditis |
Secondary causes |
SLE—systemic lupus erythematosus
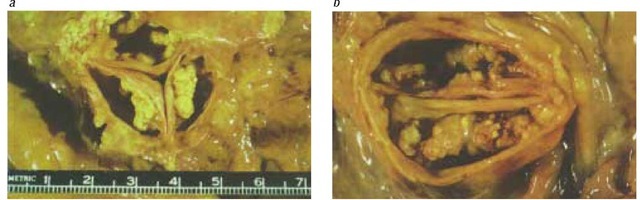
Figure 1 Pathologic specimens showing degenerative calcification of (a) a tricuspid aortic valve and (b) a congenital bicuspid valve.
Connective tissue disease
Libman-Sacks endocarditis consists of noninfected warty vegetations involving predominantly the mitral valve; it is characteristic of systemic lupus erythematosus (SLE).6 Significant regurgitation and stenosis rarely occur acutely but are seen with scarring from chronic disease. Valvular involvement in rheumatoid arthritis is common and leads to valvular thickening but is usually not of hemodynamic significance. Aortitis in ankylosing spondylitis may produce significant aortic regurgitation.
Iatrogenic causes of valvular heart disease
Iatrogenic causes include radiation therapy, the use of serotonin agonists such as methysergide, and the use of anorexiants such as fenfluramine and phentermine in combination.7,8 Radiation leads to scarring and calcification of valvular leaflets many years after the initiating radiation. The effects of both methysergide and anorexiant agents on valvular tissue often simulate rheumatic disease, but regurgitation rather than stenosis predominates as the hemodynamically severe lesion. Serotonin is also thought to play a role in the valvulopathy produced by anorexiants; the precise mechanism by which this occurs remains to be established.
Other causes of valvular heart disease
Amyloid disease causes valvular thickening but rarely causes significant stenosis. The carcinoid syndrome most often involves the valves on the right side of the heart and leads to stenosis or incompetence of the tricuspid or pulmonary valve.
Secondary involvement
Left ventricular dilatation can cause dilatation of the mitral an-nulus and, thereby, mitral regurgitation. Common secondary causes of mitral regurgitation include coronary artery disease, aortic valvular disease, and dilated cardiomyopathy. Similarly, tri-cuspid regurgitation results from right ventricular enlargement secondary to pulmonary hypertension or an atrial septal defect. Dilatation of the ascending aorta, especially involving the annulus of the aortic valve, can lead to aortic regurgitation. This condition is seen in hypertension and in aneurysms of the ascending aorta.
Assessment and Management
Valvular heart disease often remains asymptomatic for many years, but once symptoms develop, survival is reduced if the lesion is not corrected. The assessment of patients with valvular heart disease can be summarized [see Table 2]. The evaluation of symptoms can require a stress test or stress echocardiogram in addition to a careful history. Characterization of the lesions and assessment of hemodynamic severity are often possible on physical examination but are aided by additional testing, such as Doppler echocardiography and cardiac catheterization. The auscultatory findings of common valvular lesions also can be summarized [see Table 3]. Doppler echocardiography measures the flow velocity across a narrowed valve. By use of the modified Bernoulli equation, the pressure gradient (P), measured in mm Hg, may be estimated from the flow velocity (v), which is measured in m/sec: P = 4v2. Therefore, if the peak velocity recorded across the aortic valve by Doppler echocardiography is 4 m/sec, then the peak pressure gradient will be estimated as 4(42) or 64 mm Hg. The effects of valvular heart disease on chamber size and function are best assessed serially by echocardiography or, at the time of cardiac catheterization, by ventriculography. In cases of stenotic lesions, intervention is rarely required until symptoms occur. Indications for intervention in regurgitant lesions are more complex; such indications include significant symptoms or, in the absence of symptoms, increasing ventricular size, overt ventricular contractile dysfunction, or both.
Table 2 Assessment of Patients with Valvular Heart Disease
|
Parameters |
Tools |
|
Symptom severity |
History, stress testing |
|
Nature of valve lesion |
Auscultation, Doppler echocardiography |
|
Hemodynamic severity of lesion |
Physical examination, Doppler echocardiography, cardiac catheterization |
|
Effects of lesion on cardiac chamber size and function |
Echocardiography, cardiac catheterization, stress echocardiography |
|
Determination of the optimal time for intervention |
Echocardiography, stress echocardiography |
|
Selection of appropriate procedure/prosthesis |
Echocardiography |
Table 3 Auscultatory Findings Associated with Common Valve Problems
|
Lesion |
Cardiac Cycle |
Quality |
Location |
Other Sounds |
|
Aortic stenosis |
Systolic, mid-peaking to late peaking |
Harsh |
Aortic area, left sternal border, apex |
Soft S 2 , S4 |
|
Aortic regurgitation |
Diastolic, early decrescendo |
Blowing |
Left sternal border, aortic area |
— |
|
Mitral stenosis |
Diastolic, mid-peaking to late peaking, increases with atrial contraction if rhythm is normal |
Rumble |
Apex |
Opening snap, loud S1 |
|
Mitral regurgitation |
Systolic, holosystolic, late systolic with MVP, papillary muscle dysfunction |
Blowing |
Apex, axilla |
Click, soft S1, S3 |
|
Tricuspid regurgitation |
Systolic, increase with inspiration |
Blowing |
Lower left sternal border, xiphisternum |
— |
|
Pulmonary stenosis |
Systolic, mid-peaking |
Harsh |
Pulmonary area, left sternal border |
— |
MVP—mitral valve prolapse
All patients with even mild valvular heart disease require prophylaxis against endocarditis at the time of dental procedures or other procedures that can produce significant bacteremia. The prophylactic regimens recommended by the American Heart Association have been revised [see Table 4].
Despite the increase in intravascular volume, pregnancy is usually well tolerated in previously asymptomatic patients with valvular heart disease.10 During pregnancy, regurgitant lesions are better tolerated than stenosis. Prophylactic intervention to increase the valve area is recommended in patients with hemody-namically severe stenosis before pregnancy.
Patients with hemodynamically significant valvular heart disease should generally avoid participation in competitive sports. Reference should be made to the recommendations of the American College of Cardiology for more information about specific le-sions.11 Valvular heart disease is a chronic disease requiring periodic examination and follow-up, even in asymptomatic patients and in those who have had corrective surgical or other procedures. Patients with prosthetic valves should be seen at least yearly.
Specific Valvular Lesions
Mitral stenosis
Normally, the cross-sectional area of the mitral valve is at least 4 cm2. Mitral stenosis leads to a reduction in valve area and is considered severe when the valve area is less than 1 cm2. To maintain flow through the valve, left atrial pressure rises, leading to an increase in the pressure gradient across the valve and to increased pulmonary venous and capillary pressures, with resultant dyspnea. Flow through the stenotic valve is dependent on the duration of diastole. Tachycardia shortens diastole disproportionately and causes a further elevation in left atrial pressure and can precipitate symptoms even in patients with relatively mild stenosis. Elevated left atrial pressure contributes to left atrial enlargement, which in turn predisposes the patient to atrial fibrillation, atrial thrombus formation, and thromboembolism, all of which are common complications of mitral stenosis. Severe mitral stenosis is often associated with an increase in pulmonary arterial pressure, leading to right-sided heart failure and secondary tricuspid and pulmonary incompetence. In patients with severe pulmonary hypertension, cardiac output at rest is reduced; this output reduction can cause a relatively low pressure gradient across the mitral valve even in patients with severe stenosis.
Diagnosis
Clinical manifestations Mitral stenosis is often asymptomatic at presentation and for many years thereafter. Symptomatic patients often present with dyspnea, but they can also present with angina, right-sided heart failure, atrial arrhythmia, or embolism. The physical findings in mitral stenosis depend on the severity of the stenosis, the mobility of the valve, and the rhythm. The principal sign is a rumbling diastolic murmur that is best heard at the apex with the stethoscope bell. Such a murmur is accentuated by having the patient lie on the left side and by using provocative maneuvers, such as exercise to increase the heart rate. In sinus rhythm, the murmur increases in intensity with atrial contraction (presystolic accentuation). Increased severity of stenosis is associated with a longer murmur and a thrill. With a pliable valve, an opening sound (the opening snap) is heard, and the sudden closure of the stenotic valve at end diastole gives rise to a loud first heart sound that lends a tapping quality to the apex beat. When the valve calcifies and becomes less mobile, the opening snap and loud first heart sound disappear. A loud pulmonary component of the second heart sound is heard with pulmonary hypertension. The signs and symptoms of mitral stenosis are simulated by left atrial myxoma. In this condition, functional mitral stenosis results from prolapse of a mobile tumor arising from the interatrial septum into the mitral valve opening.
Imaging studies Electrocardiography can reveal left atrial enlargement if the patient is in sinus rhythm. Left atrial enlargement, mitral valve calcification, and signs of pulmonary congestion can all be present on chest x-ray. Doppler echocardiography is the test of choice in confirming the diagnosis, establishing the severity of stenosis, detecting complications, and determining the most appropriate treatment. Echocardiography also allows accurate differentiation of mitral stenosis from a left atrial myxoma.
Typically, the stenotic mitral valve leaflets are thicker and less mobile than normal. The severity of stenosis is determined by measuring the pressure gradient across the valve with Doppler echocardiography and by calculating the valve area. Mitral stenosis should be suspected if the mean gradient exceeds 5 mm Hg; the pressure can exceed 20 mm Hg in severe stenosis. Valve area is measured by tracing the smallest opening of the valve in cross section [see Figure 2]. This method is the most accurate way of defining the severity of stenosis, although it is technically demanding and sometimes impossible to perform.12 The valve area can also be calculated by Doppler echocardiography. Such evaluation is made on the basis of an empirical formula that calculates the time it takes for the pressure gradient to fall to half its initial value (the pressure half-time).
Table 4 Summary of American Heart Association Recommendations for Endocarditis Prophylaxis9
Valve area is estimated as 220 divided by the pressure half-time. Pulmonary arterial (systolic) pressure (PAP) can be determined from the tricuspid regurgitant velocity (TRv) and the estimated right atrial pressure (RAP) (usually estimated as 5 mm Hg) by the following equation: PAP = 4(TRv)2 + RAP. If the tricuspid regurgitant velocity is 3 m/sec, and RAP is estimated to be 5 mm Hg, then the estimated PAP is 4(32) + 5 = 41 mm Hg. The likelihood that the valve may be successfully dilated, either with a balloon or surgically, is estimated by use of a scoring system based on the echocardiographic appearance of the valvular leaflets and supporting structures.
Transesophageal echocardiography is more useful than trans-thoracic echocardiography in excluding atrial thrombus and determining the severity of mitral regurgitation and is usually performed if balloon valvuloplasty is contemplated. Cardiac catheterization is rarely needed to establish the diagnosis but is used to confirm the severity of stenosis. The valve gradient is the difference between the left atrial pressure or the pulmonary arterial wedge pressure and the left ventricular diastolic pressure. Valve area can be calculated from the pressure gradient and the cardiac output.
Treatment
Once symptoms develop in mitral stenosis, the chance of survival decreases without surgical or balloon dilatation or valve replacement. In the absence of symptoms, management is directed at preventing recurrence of rheumatic fever.13
Medical therapy Patients in atrial fibrillation require heart-rate control with a beta blocker (e.g., atenolol, 50 mg q.d.), digoxin (0.125 to 0.25 mg q.d.), or both. Systemic anticoagulation with warfarin is definitely indicated to prevent thromboembolism when (1) atrial fibrillation is present, (2) there is a history of embolism, or (3) a thrombus is detected in the atrium. Anticoagulation should be considered for patients with paroxysmal atrial fibrillation, a dilated left atrium (> 50 mm in diameter on echocardiography), or severe atrial stasis (as evidenced by swirling echoes or smoke in the left atrium on echocardiography).14 Regarding symptomatic patients for whom surgical intervention poses a relatively high risk, the judicious use of diuretics and drugs to control heart rate (i.e., digoxin, calcium channel blockers, or beta blockers) may allow symptomatic relief without the need for surgical intervention.
Surgical intervention Intervention to increase valve area is indicated before the onset of symptoms of dyspnea in the following patients: women with severe stenosis who wish to become pregnant but are unlikely to tolerate the volume load of pregnancy, patients who experience recurrent thromboembolic events, and patients who have severe pulmonary hypertension. A number of interventions are currently available to increase the valve area in mitral stenosis. These interventions include percutaneous balloon valvuloplasty, performed in the cardiac catheterization laboratory; surgical commissurotomy; and replacement of the mitral valve with a prosthesis.15
Balloon valvuloplasty is performed by inflating a specially designed balloon catheter in the mitral orifice to split the fused commissures. Excellent symptomatic relief is obtained in suitable patients.16 This intervention is currently the initial choice in mitral stenosis. Typically, the mitral valve area doubles in size from 1.0 to 2.0 cm2, with a concomitant reduction in the pressure gradient [see Figure 3]. Complications of balloon mitral valvuloplasty include severe mitral regurgitation (3%), thromboembolism (3%), and residual atrial septal defect with significant shunting (10% to 20%). Mortality associated with the procedure is less than 1%.17,18 Contraindications to balloon mitral valvuloplasty include significant mitral regurgitation, which will likely increase after balloon inflation; left atrial thrombus, which can be dislodged at the time of the procedure; and significant subvalvular involvement or leaflet calcification, each of which increases the risk of complications and limits the degree of dilatation produced.19 In pregnant patients with symptomatically severe mitral stenosis that has not responded to conservative measures such as bed rest and heart-rate control, balloon valvuloplasty is the technique of choice to increase the valve area.20
Surgical commissurotomy is now usually performed under direct vision after cardiopulmonary bypass.
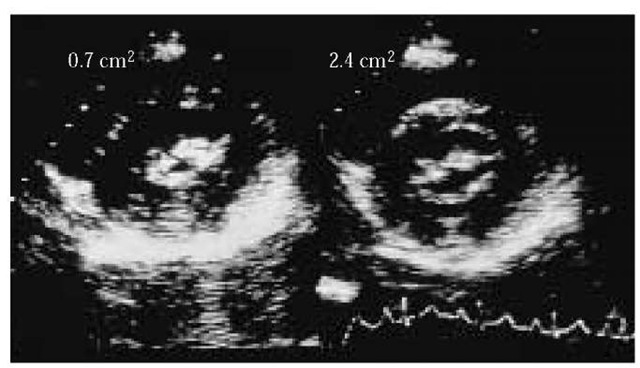
Figure 2 Two-dimensional echocardiography parasternal short-axis image of a mitral valve before (left) and after (right) percutaneous balloon mitral valvuloplasty. The valve area is estimated by planimetry and increases from 0.7 cm2 before valvuloplasty to 2.4 cm2 after valvuloplasty.
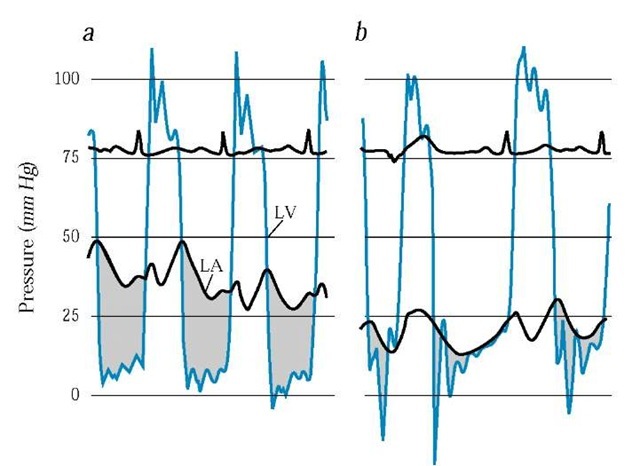
Figure 3 Simultaneous left atrial pressure (LA, black line) and left ventricular pressure (LV, blue line) are shown (a) before and (b) after percutaneous mitral valvuloplasty. The shaded area shows the pressure gradient across the mitral valve; the pressure falls after valvuloplasty.
Surgical commissuroto-my may be feasible when balloon valvuloplasty is impossible, such as in patients with significant mitral regurgitation, subvalvu-lar stenosis, or atrial thrombus. A number of studies comparing surgical commissurotomy with balloon commissurotomy have shown equivalent immediate and medium-term (3- to 4-year) results regarding increase in valve area, improvement in symptoms, and freedom from repeat intervention in appropriately selected patients.19 However, commissurotomy, whether effected by a balloon or surgically, is a palliative procedure, and in most cases further intervention is eventually required. Repeat commis-surotomy is sometimes feasible; but most often, mitral valve replacement is also necessary.21
A prosthetic replacement is indicated if the valve is heavily scarred or calcified or if severe mitral regurgitation is present. Morbidity and mortality are higher with prosthetic replacement than with either surgical or balloon commissurotomy.