Management
Thyroid Hormone Therapy
Levothyroxine sodium (thyroxine) is the treatment of choice for patients with hypothyroidism. Thyroxine is well absorbed by the proximal small bowel. Thyroxine circulates with a 7-day half-life because of plasma protein binding, and it is metabolized in target tissues, in part by deiodination to T3. Its long half-life permits a single daily dose; its conversion to T3 in target tissues mimics normal physiology. The multiple dose strengths available in North America facilitate precise dose titration. Nonetheless, thyroxine and other thyroid hormone preparations have narrow therapeutic indexes and hence have the potential for adverse reactions with even modest overtreatment. Several studies examining the adequacy of thyroid hormone therapy in large populations and in patients in generalist and specialty practices have found that one fifth of patients with treated hypothy-roidism are receiving an inadequate dose and one fifth an excessive dose.39
Dosing considerations and drug interactions The optimal thyroxine dosage for hypothyroid patients is related to body weight. In adults, this is approximately 1.8 ^g/kg/day.40 Elderly patients, whose metabolic clearance of thyroxine is reduced, have a lower dosage requirement of 0.5 ^g/kg/day. The thyrox-ine dose is usually higher in patients who have undergone thy-roidectomy than in patients with autoimmune thyroiditis, who often have residual functioning thyroid tissue. Thyroxine absorption can be decreased in patients with malabsorption from gastrointestinal disorders or previous small bowel bypass surgery. Several mineral supplements, medications, and dietary constituents can interfere with thyroxine absorption; these include iron, calcium carbonate, cholestyramine, aluminum hydroxide gel, sucralfate, soy, and perhaps dietary fiber. Metabolism of thyroxine is accelerated in the nephrotic syndrome, in other severe systemic illnesses, and with the use of phenobarbi-tal, phenytoin, carbamazepine, and rifampin. In 75% of pregnant women, the thyroxine dose requirement is increased by 50% to 100%.41 Postmenopausal hormone replacement therapy increases the required thyroxine dose in 35% of women.
Patient noncompliance is the most common cause of inadequate thyroxine therapy. Several observations should raise suspicion that a patient is not taking thyroxine faithfully: the apparent thyroxine dose requirement is higher than expected; thyroid function test results vary without correlation with prescribed thyroxine doses; and the serum TSH concentration is elevated, yet the serum free T4 level is in the mid- to high-normal range, reflecting improved compliance immediately before testing.
Thyroxine treatment should typically start with a dosage at the lower end of the anticipated requirement (e.g., 125 ^g/day in a 70 kg adult). In otherwise healthy younger patients, there is no need to titrate the dose upward from a very low starting dose. Laboratory monitoring of treated hypothyroid patients should be performed 4 to 6 weeks after starting a new thyroxine dose or tablet formulation; thereafter, it should be performed annually. It should also be performed whenever a patient’s symptoms suggest thyroid hormone deficiency or excess. The goal for most patients is to restore the TSH level to the lower half of the normal range (i.e., 1.0 to 2.0 ^U/L). In patients with central hypothy-roidism, the serum free T4 concentration must be monitored; treatment should usually be targeted for a concentration in the upper half of the normal range.
Metabolism of certain other drugs can be affected by the hy-pothyroid state and by the initiation of thyroxine treatment. Hy-pothyroid patients may have increased sensitivity to anesthetic and sedative agents. Reduced digoxin clearance and drug distribution volume may predispose patients to toxicity. Sensitivity to warfarin may be decreased because of slowed metabolism of vitamin K-dependent clotting factors, and restoring euthyroidism can increase the required warfarin dose.
Adverse reactions to thyroid hormone therapy Adverse reactions to thyroxine overtreatment include symptomatic thyro-toxicosis and subclinical thyrotoxicosis with increased risks of bone loss and atrial tachyarrhythmias.43,44 The predisposition to osteoporosis is principally in postmenopausal women. Atrial fibrillation is more common in patients 60 years of age or older. Both of these complications have been shown to occur when the serum TSH concentration is suppressed to less than 0.1 ^U/L.
Complications can also arise from restoring euthyroidism, particularly in patients with underlying ischemic heart disease45 (see below) and borderline adrenal cortical insufficiency. Concomitant thyroid and adrenal gland failure can occur in hypopi-tuitarism and in the type 2 polyendocrine failure syndrome (Schmidt syndrome), which is marked by autoimmune thyroidi-tis and idiopathic adrenal insufficiency.
A few patients experience acute sympathomimetic symptoms soon after institution of thyroxine treatment. This syndrome is poorly understood; it can be circumvented by reducing the thy-roxine dose to a very low level and advancing it slowly.
Transient scalp hair loss may occur during first few weeks of thyroxine replacement therapy. Patients can be assured that this phenomenon is temporary. Treatment of hypothyroidism sometimes reveals an underlying urticarial disorder, but true allergy to thyroxine formulations has not been well documented.
Special Therapeutic Issues
Hypothyroid patients with ischemic heart disease Because thyroid hormone has positive inotropic and chronotropic effects, thyroid hormone therapy can exacerbate myocardial ischemia in hypothyroid patients with underlying coronary artery disease. In such patients, thyroxine therapy should be initiated at a low dosage (e.g., 25 ^g/day) and titrated upward in increments of 12.5 to 25 ^g every 4 to 6 weeks. Patients should be monitored vigilantly with clinical assessments and electrocardiography. Deliberate suboptimal dosing, which was previously advocated to limit myocardial oxygen demand, has been shown to actually increase the risk of progressive coronary atherosclerosis. Beta-blocker therapy should sometimes be initiated or intensified when thyroxine therapy is initiated. Hypothyroid patients who experience worsening myocardial ischemia despite these precautions can undergo coronary angioplasty and even surgical bypass grafting with minimal or no increased perioperative risk.
Mild hypothyroidism Whether to identify and treat patients with mild hypothyroidism, defined by an elevated serum TSH level with a normal free T4 level, is controversial. There is agreement that mild hypothyroidism is highly prevalent, particularly in older women, and that clinical diagnosis is inaccurate. Diagnostic serum TSH testing and thyroxine treatment of mild hypothyroidism are clearly effective and are relatively safe and inexpensive. The outstanding issue is whether mild hypothy-roidism causes clinical consequences that are important enough to justify widespread screening and therapy.48 Proponents of detection and treatment argue that it prevents progression to overt hypothyroidism in affected patients, particularly those whose serum TSH concentration is greater than 10 ^U/L, who are 65 years of age or older, or who have thyroid autoantibodies, indicating underlying autoimmune thyroiditis. Advocates believe that treatment of mild hypothyroidism may reduce the risk of future atherosclerotic cardiovascular disease. They hold this view on the basis of the following observations: affected patients have higher mean cholesterol levels; most studies have shown that TSH-normalizing thyroxine therapy lowers serum total cholesterol and LDL cholesterol concentrations49; and some epi-demiologic studies have found that persons with mild hypothyroidism have a higher risk of atherosclerotic cardiovascular dis-ease.50 Some proponents are persuaded by four small, controlled, double-blind trials that showed that thyroxine therapy was more effective than placebo in improving symptoms and neuropsy-chologic performance in patients with mild hypothyroidism.51 On the basis of these studies, two decision and cost-effectiveness models suggested that the cost-effectiveness of screening for and treating mild hypothyroidism is comparable to that of other widely accepted preventive medicine strategies.52,53 On the other hand, opponents of screening and treatment of mild hypothy-roidism point out that these putative benefits have not been rigorously confirmed by large, randomized, controlled trials.54 When physicians do recommend treatment for patients with mild hypothyroidism, the thyroxine dosage is typically lower than that for overt hypothyroidism—0.5 ^.g/kg/day.
Residual hypothyroid symptoms and T3 therapy Compared with euthyroid patients, hypothyroid patients more often have constitutional and neuropsychological complaints, even when serum TSH measurements suggest adequate treatment.55 This observation may represent only ascertainment bias (i.e., symptomatic patients seeking medical care are more likely to be diagnosed and treated for hypothyroidism). However, it has been postulated that the presence of residual symptoms in thy-roxine-treated patients reflects a failure to replace the small amount of T3 normally secreted by the thyroid gland. Four clinical trials in which a fraction of the thyroxine dose was replaced with a small dose of T3 failed to confirm an earlier report of significant improvement with combination thyroxine/T3 therapy.56 Combination therapy has the disadvantages of a fluctuating and supraphysiologic T3 level, a greater risk of iatrogenic thyrotoxi-cosis, and increased complexity and expense. Treatment with desiccated thyroid, a biologic preparation that also contains both T4 and T3, has the same disadvantages.
Complications
Severe hypothyroidism (myxedema) can become complicated by multiple organ system failure when it is profound and prolonged, especially in elderly patients who have other cardiac, pulmonary, neurologic, renal, and infectious diseases. Myxede-ma coma, the most severe expression of hypothyroidism, is associated with substantial mortality. Such complications of thyroid hormone deficiency can be prevented with sustained thyroxine therapy. In newly diagnosed patients, preventive measures also include giving special attention to other potentially provocative medical conditions (e.g., heart failure, renal failure, pneumonia) and medications—particularly sedative, anesthetic, and analgesic medications that suppress ventilatory drive and other central nervous system functions.
Treatment of complicated hypothyroidism includes thyroid hormone replacement and aggressive management of organ system complications that can be present. Two thyroid hormone regimens have proven efficacy for myxedema coma: (1) thyroxine in a full replacement dose (1.8 ^.g/kg/day), with or without a 500 ^.g loading dose to replete the normal body thyroxine pool57; and (2) T3 in divided doses, advocated because of the impaired T4-to-T3 conversion that occurs in critically ill patients. No trial has rigorously compared these regimens, but one small retrospective study found a higher mortality in T3-treated patients.58
Prognosis
The prognosis for hypothyroid patients who are properly treated with thyroxine should be excellent. However, discontinuance of thyroid hormone therapy predictably leads to recurrent hypothyroidism, with its potential for serious complications in the elderly. This occurs most often in settings of social neglect, poor access to health care, and associated neuropsychological impairment. Lesser degrees of suboptimal therapy are also associated with long-term risks. Inadequately treated patients may have increased risk of atherosclerotic cardiovascular disease, and iatrogenic thyrotoxicosis can predispose patients to osteoporosis and atrial tachyarrhythmias.
Patients with autoimmune thyroiditis, the most common cause of hypothyroidism, are at risk for certain associated conditions, for which they should be monitored. Pernicious anemia and gastric achlorhydria with consequent iron and calcium mal-absorption affect 3% and 25% of autoimmune thyroiditis patients, respectively. Much less commonly, other autoimmune diseases (e.g., Sjogren syndrome and systemic sclerosis), endocrine deficiency states (adrenal insufficiency, type 1 diabetes, hypoparathyroidism, and hypogonadism), and primary thyroid lymphoma can occur.
Thyrotoxicosis
Epidemiology
The alert clinician will diagnose thyrotoxicosis several times each year. NHANES III found thyrotoxicosis in 0.5% of a surveyed cohort that reflected the demographics of the United States adult population.1 Three disorders account for the majority of cases: diffuse toxic goiter (Graves disease), toxic nodular goiter, and iatrogenic thyrotoxicosis in thyroid hormone-treated patients. The incidence of Graves disease in one United Kingdom community survey was one to two cases per 1,000 population annually; 2.7% of women and 0.2% of men had Graves disease or a history of Graves disease.59 The highest incidence of Graves disease is in women 30 to 60 years of age, but the disease can affect persons of virtually any age, from neonates to the very elderly. Toxic adenoma and toxic multinodular goiter are more common causes of thyrotoxicosis than Graves disease in regions where dietary iodine deficiency is prevalent; in women; and in older patients.60 Iatrogenic thyrotoxicosis has been reported in approximately 20% of thyroid hormone-treated patients.
Etiology, genetics, and pathogenesis
Thyrotoxicosis can be divided into three etiologic categories: abnormal stimulation of the thyroid gland, thyroid gland autonomy, and gland inflammation with unregulated thyroid hormone release. Each of these categories includes several diseases [see Table 2].
Graves Disease (Diffuse Toxic Goiter)
There is compelling evidence that there is a genetic predisposition to Graves disease, that the incidence is higher in women, that unknown environmental factors are involved in its initiation, and that gland stimulation by antibodies against the TSH receptor is the immediate precipitant of the condition. Identical twins and some families show increased incidences of Graves disease.62 The condition has been genetically linked to certain MHC components (e.g., HLA-B8 and HLA-DR3), which are on the surface of cells that present antigenic peptide epitopes to T cell receptors. One theory is that certain HLA-DR molecules may be better able to present TSH receptor epitopes, inciting autoimmunity. Anoth-er hypothesis is that these HLA-DR recognition sequences are involved in aberrant thymic T cell selection for tolerance.
Table 2 Etiologic Classification of Thyrotoxicosis
|
Cause |
Individual Diseases |
|
Abnormal stimulation of the thyroid gland |
Graves disease |
|
hCG-mediated thyrotoxicosis TSH-mediated thyrotoxicosis |
|
|
Thyroid gland autonomy |
Toxic adenoma |
|
Toxic multinodular goiter |
|
|
Congenital thyrotoxicosis |
|
|
Iodine-induced hyperthyroidism |
|
|
Thyroid cancer-related thyrotoxicosis |
|
|
Gland inflammation with unregulated thyroid hormone release |
Subacute (de Quervain) thyroiditis |
|
Lymphocytic thyroiditis |
|
|
Amiodarone-induced thyrotoxicosis, type 2 |
|
|
Acute thyroiditis |
hCG—human chorionic gonadotropin
Graves disease has also been linked to polymorphisms in the gene encoding CTLA-4, a T cell receptor important for interaction with antigen-presenting cells.63 In whites, a susceptibility locus for Graves disease has been identified on chromosome 20q11.
Several environmental factors have been implicated in the initiation of Graves disease. These include stressful life events, smoking, large amounts of dietary iodine, and preceding infection with certain bacterial agents that have been postulated to induce molecular mimicry. Radiation injury to the thyroid gland may increase the risk of the condition, possibly because of increased TSH receptor exposure and immunoreactivity.
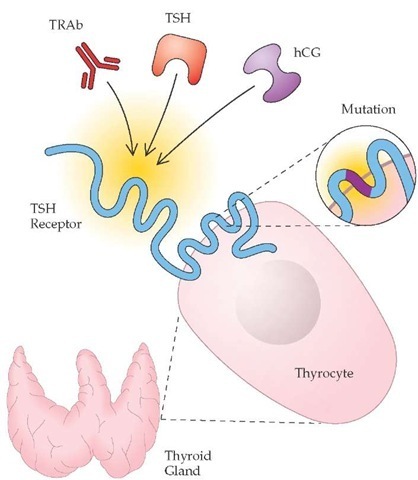
Whatever the underlying genetic and environmental factors, the vast majority of Graves disease patients have detectable antibodies that are directed against the TSH receptor and are capable of stimulating it64 [see Figure 2]. Assays using thyroid cells or their membranes can detect circulating TSH receptor autoantibody species in 70% to 90% of patients with Graves disease. These au-toantibodies are capable of stimulating intracellular cyclic aden-osine monophosphate production (thyroid-stimulating immu-noglobulins [TSI]), inhibiting TSH receptor activation (TSH receptor inhibitory immunoglobulins), and inhibiting the binding of TSH to its receptor (TSH receptor-binding inhibitory immu-noglobulins [TBII]).
Toxic Adenoma and Toxic Multinodular Goiter
Solitary and multiple thyroid adenomas and diffusely hyper-plastic thyroid tissue possess a growth advantage, and their constituent thyrocytes sometimes produce thyroid hormones autonomously (i.e., without regard to TSH regulation). These hy-perplastic and neoplastic conditions cause hyperthyroidism when the mass and efficiency of functioning thyroid tissue are great enough to generate hormone excess in target tissues, including suppression of endogenous pituitary TSH production and function of extranodular thyroid tissue.65 Both genetic and environmental factors are involved in the development of this autonomous function. A twin study showed that genetic factors could account for 82% of the predisposition to nodular goiter, and familial multinodular goiter has been linked to a gene locus on chromosome 14q.66 At the same time, environmental factors (e.g., dietary iodine deficiency, goitrogens, and radiation exposure) also clearly predispose to the development of autonomously functioning thyroid tissue. Genetic and environment factors promote thyroid tissue growth by activating intraglandular growth factors (e.g., insulinlike growth factor and epidermal growth factor receptor)67 and signaling pathway proteins (e.g., Gsa and ras).
Figure 2 Certain forms of hyperthyroidism result from deranged physiologic activation of the thyroid-stimulating hormone (TSH) receptor. In Graves disease, TSH receptor autoantibodies (TRAb) bind the TSH receptor. In patients with TSH-secreting pituitary tumors, the autonomously secreted TSH overstimulates the receptor. In molar pregnancy and choriocarcinoma, high concentrations and aberrant forms of human chorionic gonadotropin (hCG) can activate the TSH receptor. In some patients with toxic adenomas, a constitutively activating somatic mutation of the TSH receptor results in autonomous secretion of thyroid hormone.
Constitutively activating somatic mutations of the TSH receptor and Gsa itself have been described in 25% to 80% of toxic adenomas, more commonly in patients from regions where dietary iodine deficiency is prevalent.68
Iodine-Induced Hyperthyroidism
Iodine is both a substrate and a physiologic regulator of thyroid hormone synthesis. Excessive iodine intake normally inhibits thyroid hormone production by reducing the trapping of inorganic iodide and its oxidation into an organic form (organifi-cation) and by thyroid hormone release. At the same time, exposure to pharmacologic amounts of iodine (typically 1,000-fold more than the physiologic requirement of 150 ^g/day) can cause hyperthyroidism, a condition termed the Jod-Basedow effect. Patients with hyperplastic and benign neoplastic thyroid conditions and those with latent Graves disease are particularly vulnerable to iodine-induced hyperthyroidism. Epidemics of thyro-toxicosis have repeatedly been observed when iodine supplementation is instituted in regions of previous dietary iodine deficiency.69 However, iodine-induced hyperthyroidism can also occur in patients from iodine-sufficient environments whose thyroid glands are apparently normal, especially when excess iodine exposure is substantial and sustained, as it is with long-term amiodarone therapy.70 The precise molecular and biochemical basis for iodine-induced hyperthyroidism is poorly understood. Iodine-induced hyperthyroidism is typically transient, lasting only a few weeks, but more prolonged thyroid dysfunction can occur when iodine exposure is prolonged, as occurs with the lipid-soluble drug amiodarone and with myelographic radiocontrast agents.
Thyroiditis
Inflammation of thyroid tissue caused by infectious diseases, autoimmune processes, or pharmacologic toxicity can cause thy-rocyte death, disruption of follicular architecture, and unregulated leakage of thyroid hormones from the gland into the circulation, resulting in thyrotoxicosis71 [see Table 3]. Thyroiditis-related thyrotoxicosis is typically self-limited, lasting 2 to 8 weeks, with spontaneous resolution once glandular stores of thyroid hormone are exhausted. A comparable period of transient hypothy-roidism often follows because of lingering impairment of thyroid hormone synthesis, but most patients ultimately become euthyroid [see Figure 3].
Amiodarone-Induced Thyrotoxicosis
The iodine-containing antiarrhythmic agent amiodarone can cause thyrotoxicosis by two mechanisms.72 Type 1 amiodarone-induced thyrotoxicosis is caused by iodine, whereas type 2 amiodarone-induced thyrotoxicosis is the result of gland inflammation. Both forms can be severe, prolonged, and life-threatening, particularly because affected patients have underlying cardiac disease.