Hereditary Hypercoagulable States
Antithrombin Deficiency
Epidemiology and Etiology
The frequency of symptomatic inherited AT deficiency in the general population has been estimated to be approximately 1 per 2,000 people.5 The deficiency is transmitted in an autosomal dominant pattern. Homozygous AT deficiency has not been reported, presumably because the condition is incompatible with normal fetal development.
There are two types of inherited AT deficiency. Type I is quantitative, as measured by antigenic and functional assays. A large number of molecular mutations have been characterized in type I AT deficiency, including partial gene deletions and sin-gle-nucleotide substitutions that cause nonsense or missense mutations leading to premature stop signals in the protein-translation process.
Type II deficiency is qualitative; plasma levels of AT antigen are normal. The underlying defect is generally a single nu-cleotide change that causes missense mutations, giving rise to a dysfunctional protein. Many of these proteins have decreased affinity for heparin binding.
In rare cases, AT deficiency is acquired. This condition may occur after administration of intravenous heparin for more than 3 days or after asparaginase therapy. It may also develop in patients with disseminated intravascular coagulation (DIC), severe liver disease, or the nephrotic syndrome.
Pathophysiology
AT inactivates factor Xa and thrombin by forming a stable stoichiometric complex with each of them. AT is present in sufficient amounts in plasma to inactivate all the thrombin formed in a given plasma volume, but it does so slowly unless it is activated by endothelial cell surface heparan sulfate or by administered heparin [see 5:XIII Hemorrhagic Disorders]. Patients with hereditary AT deficiency have evidence of continuous factor X activation and thrombin generation (as supported by elevated plasma levels of prothrombin fragment F1.2) even when they are clinically asymptomatic.
Clinical Presentation
Patients with AT deficiency show an increased incidence of venous thrombosis, usually triggered by a prothrombotic stimulus such as surgery, infection, immobilization, or trauma. This association suggests that the superimposition of a prothrombot-ic stimulus on an underlying subclinical hypercoagulable state leads to clinical thrombosis.
Typical clinical presentations are DVT of the legs, pulmonary embolism, and occasionally mesenteric vein thrombosis. There is no convincing evidence to suggest that AT deficiency increases the risk of arterial thrombosis.6
Affected patients usually have a family history of recurrent thromboses, generally beginning in youth and often associated with surgery or trauma. Pregnancy and the use of oral contraceptives also increase the risk of thromboses in AT-deficient patients. The tendency to thrombosis increases with advancing age: by age 50, only 10% of AT-deficient patients are free of symptoms.
Diagnosis
The AT level should be determined by a functional assay rather than an antigenic assay, so that both type I and type II deficiency can be evaluated. Patients with AT deficiency have a surprisingly modest reduction in the protein: values measured by both bioassay and immunoassay range from 25% to 60% of normal in type I disease.
Treatment
Study of a large AT-deficient kindred indicates that long-term anticoagulant prophylaxis is not warranted in asymptomatic carriers of this deficiency.7 Asymptomatic carriers should receive prophylactic anticoagulation only in situations known to increase the risk of thrombogenesis, such as abdominal surgery.7 Once such patients have experienced a thrombotic event, however, they probably require lifelong warfarin therapy. Warfarin is the mainstay of long-term therapy for patients with AT deficiency and recurrent thromboembolism.
Acute episodes of thrombosis must be treated with heparin. Because AT deficiency may render heparin relatively ineffective, the physician should be alert to heparin resistance. In patients receiving unfractionated heparin, resistance is manifested by minimal prolongation of the partial thromboplastin time (PTT) despite the administration of therapeutic doses. If low-molecular-weight heparin (LMWH) is used, as is commonly the case, the level of anti-factor Xa (anti-FXa) should be checked to ensure that a therapeutic anticoagulant effect is achieved [see 5:XIl Hemostasis and Its Regulation].
If heparin resistance occurs despite increased doses of hep-arin, heparin plus purified AT concentrates or fresh frozen plasma should be given. AT has a half-life of about 60 hours. These preparations can be used to carry an AT-deficient patient through surgery or delivery and should bring the AT level up to nearly 100%, depending on the patient’s baseline AT level. The AT level should be checked and the infusion repeated at 24-hour intervals to maintain a normal AT level for 5 to 7 days after delivery or surgery.
Pregnancy in an AT-deficient patient is difficult to manage. Because warfarin may cause fetal malformations and neonatal hemorrhage, patients should be treated with full-dose unfrac-tionated heparin or LMWH; those receiving LMWH should be switched to unfractionated heparin 1 to 2 weeks before delivery so that rapid reversal of anticoagulation, if necessary, can be more easily attained. If a therapeutic effect cannot be achieved (as measured by the PTT with unfractionated heparin or the anti-FXa level with LMWH), an AT infusion can be given.8 Generally, this is not necessary. Anticoagulation should be promptly reinstituted after delivery.
Protein c and Protein S Deficiency
Pathophysiology and Clinical Presentation
Deficiency or defect in protein C or protein S results in a loss of ability to inactivate excess factor Villa and factor Va, the two major cofactors that regulate amplification of the clotting cascade. Protein C levels are low in patients with DIC and liver disease, probably because the activation of hemostasis consumes this factor [see 5:Xlll Hemorrhagic Disorders].
Homozygous protein C deficiency causes lethal thrombosis in infants. Heterozygous protein C deficiency probably occurs with a prevalence of 1 per 200 to 300 in the general population. Clinical expression of heterozygous protein C deficiency varies: many persons with heterozygous deficiency, as well as persons with low-normal protein C levels from other causes, do not experience thrombosis,9 whereas other patients with heterozygous deficiency exhibit a definite tendency toward venous thrombosis even though their protein C levels are 40% to 50% of normal. This phenotypic variability suggests multiple gene interactions and supports the hypothesis that clinical thrombosis in such patients may result from a combination of protein C deficiency and one or more other prothrombotic mutations.10 Cerebral venous thrombosis presumably accounts for cases of cerebral hem-orrhagic infarction that occur in young adults with protein C deficiency.
Deficiency of protein S also leads to venous thrombosis, including mesenteric vein thrombosis. Pregnancy and the use of oral contraceptives lower the protein S level, which may account for some cases of thromboembolism that occur under such cir-cumstances.11 Acquired protein S deficiency also occurs in patients with the nephrotic syndrome, who lose protein S in urine.12 Case reports have associated protein S deficiency with warfarin-induced skin necrosis.13
Diagnosis
Functional and antigenic assays for protein C and protein S are now available in most coagulation laboratories. Functional assays are preferable for diagnosis. It is important to measure free protein S because some patients who have low free protein S levels have normal or borderline total protein S levels. Coagulation assays for protein C and protein S can give falsely low values in patients with factor V Leiden.
Treatment
Warfarin is the treatment of choice for preventing thrombosis, even though it lowers protein C levels still further. Because the half-life of protein C is only 6 to 7 hours, much shorter than that of prothrombin and factor X, a period of enhanced hypercoagulability follows initiation of warfarin therapy in patients with protein C deficiency. Heparin should be given along with warfarin during the initiation of anticoagulation; it can be withdrawn afterward. Warfarin-induced skin necrosis is a rare complication of anticoagulation therapy.
Factor V Leiden
Epidemiology and Etiology
Factor V Leiden is a mutated form of factor V (first identified by researchers in Leiden, The Netherlands) that, once activated, is relatively resistant to the anticoagulant effects of activated protein C (APC). The defect is transmitted as an autosomal dominant trait. Approximately 5% of the general white population is heterozygous for factor V Leiden; the defect is almost absent in other ethnic groups.15 Factor V Leiden is now considered to be the most common hereditary hypercoagulable state. Its prevalence in patients with thrombophilia is as high as 20% to 50%.16 In a large cohort study of unselected patients with a first episode of symptomatic DVT, factor V Leiden was found in 16% of patients.17 In women who have thrombosis while taking oral contraceptives, the frequency of factor V Leiden is about 23%.18 The relative risk of DVT in a factor V Leiden homozygote (estimated incidence, 0.5% to 1% a year) is approximately 80-fold higher than in a normal person.19 The risk of thrombosis in persons who are heterozygous for factor V Leiden is estimated to be fourfold to eightfold higher than that in normal persons; the relative risk increases to more than 30-fold when factor V Leiden is combined with oral contraceptive use. The absolute risk of thrombosis, however, is low.20 Association of factor V Leiden with deficiencies of protein C, protein S, or AT has been reported in some families.21-23 Overall, although factor V Leiden is highly prevalent, it is a relatively weak risk factor for thrombosis.
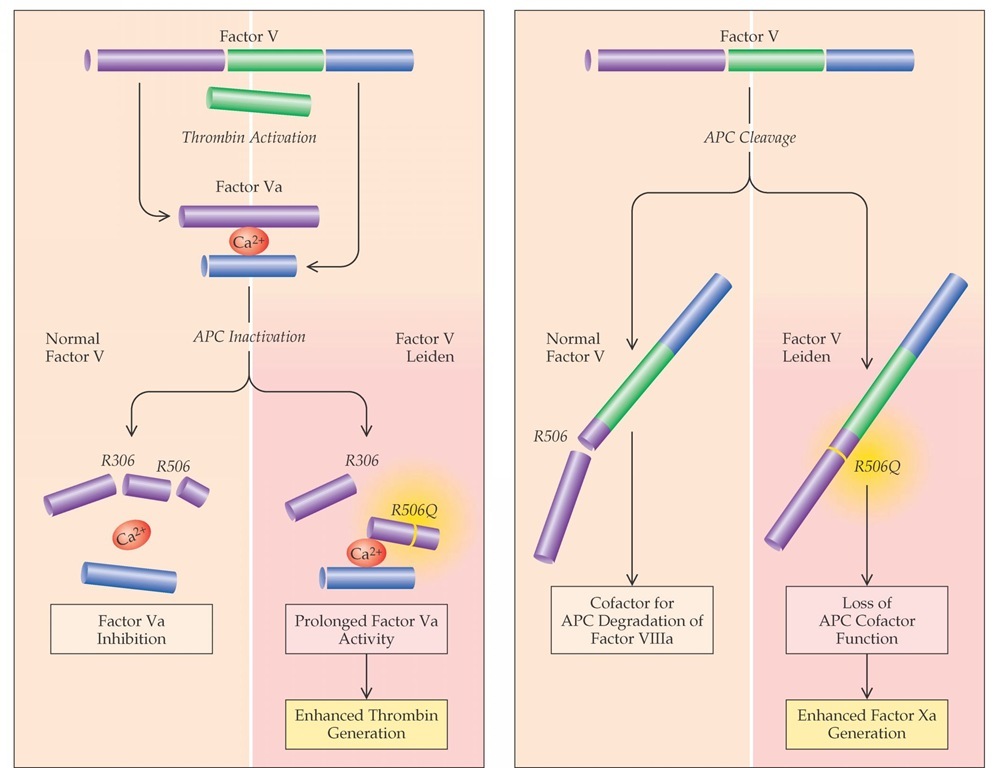
Figure 1 Degradation of thrombin-activated factor V Leiden by activated protein C (APC) is significantly slower than that of normal activated factor V (factor Va), which leads to enhanced thrombin generation (left). Recent evidence suggests that normal factor V, together with protein S, serves as a cofactor of APC in the inhibition of factor VIIIa (right). This APC cofactor function of factor V requires the cleavage of factor V by APC at arginine 506; therefore, factor V Leiden has a poor cofactor function.
Approximately 5% of cases associated with inherited resistance to APC are attributable to other mutations and defects.24-26 Conditions such as factor VIII elevation, pregnancy, oral contraceptive use, and lupus anticoagulant may result in APC resis-tance.27 APC resistance that is not caused by factor V Leiden may be a risk factor for stroke28,29 and venous thrombosis.30,31 The overall risk of venous thrombosis from APC resistance is similar to or less than that posed by factor V Leiden.
Pathophysiology
Resistance to the anticoagulant effects of APC is caused by a specific mutation in factor V (factor V Leiden or factor V R506Q) that results from a single-nucleotide substitution that leads to the replacement of arginine with glutamine at position 506.32 Arginine 506 is located at one of the two major APC cleavage sites of activated factor V. Activated factor V Leiden expresses normal procoagulant activity, but its degradation by APC is approximately 10 times slower than that of normal activated factor V (factor Va). This slowing leads to increased thrombin generation.33 In addition, recent evidence suggests that factor V (but not factor Va), together with protein S, serves as a cofactor of APC in the inhibition of the factor VIIIa/factor IXa complex and that factor V Leiden has a poor APC cofactor function [see Figure 1].
Clinical Presentation
Clinical manifestations of factor V Leiden are similar to those of deficiencies of AT, protein C, and protein S—mainly, venous thrombosis. However, the first thrombotic manifestation in factor V Leiden often occurs later than in the other hereditary thrombophilic states. Approximately 25% of apparently healthy men older than 60 years who experience a first episode of venous thrombosis have factor V Leiden.34 There are conflicting data on whether factor V Leiden is associated with an increased risk of recurrent deep vein thrombosis. Several studies reported a slightly enhanced recurrence risk (twofold to fourfold), but more recent studies have shown that the risk of recurrence is similar to that in persons without the mutation.
Diagnosis
Factor V Leiden can be identified rapidly and precisely with simple DNA-based tests. These tests allow the diagnosis to be made in patients receiving anticoagulation therapy with warfarin and in those who have coexisting antiphospholipid antibodies. Because factor V Leiden is not the sole cause of APC resistance, it may be worthwhile to pursue the diagnosis with an APC-resistance test in selected cases.
Treatment
Management of factor V Leiden is similar to that of AT, protein C, and protein S deficiencies. Patients with a first episode of venous thrombosis should receive anticoagulation therapy for 6 months. Thereafter, they should be given prophylactic anticoag-ulation therapy in situations known to provoke thrombosis. Long-term anticoagulation should be considered in patients with recurrent thrombosis.
Young women known to be factor V Leiden carriers should avoid the use of oral contraceptives, which increases the relative risk of thrombosis (although the risk remains low in terms of absolute incidence). The optimal treatment of carriers during pregnancy has not been established. The rate of venous thromboem-bolism is low, about 2% without thrombosis prophylaxis.20 My practice is not to use thrombosis prophylaxis routinely during pregnancy, but I will consider postpartum prophylaxis for 6 weeks, especially when the family history of thrombosis is strong. Routine screening of family members of patients with factor V Leiden is not cost-effective.
Prothrombin Gene Mutation 20210A
A G-to-A mutation at nucleotide position 20210 in the 3′ untranslated region of the prothrombin gene is associated with an increased incidence of venous thrombosis. The prevalence of the mutation in healthy persons is about 2.3%. Like factor V Leiden, this mutation is very rare in Asians and Africans. Unlike factor V Leiden, it is more common in southern Europeans than in northern Europeans.39 The relative risk of thrombosis in persons with this mutation is 2.8, which is similar to the relative risk in those with factor V Leiden.40 The mutation can be found in up to 18% of patients with thrombosis and family histories of thrombosis. The most common presentation is DVT of the lower extremities. Prospective studies have not shown an increased risk of recurrent DVT in patients with this mutation.41 However, carriers who are heterozygous for both factor V Leiden and the pro-thrombin mutation have a higher risk of recurrent thrombosis.35 The combination of oral-contraceptive use and the prothrombin gene mutation is associated with an increased incidence of cerebral vein thrombosis in young women.42
Hyperhomocysteinemia
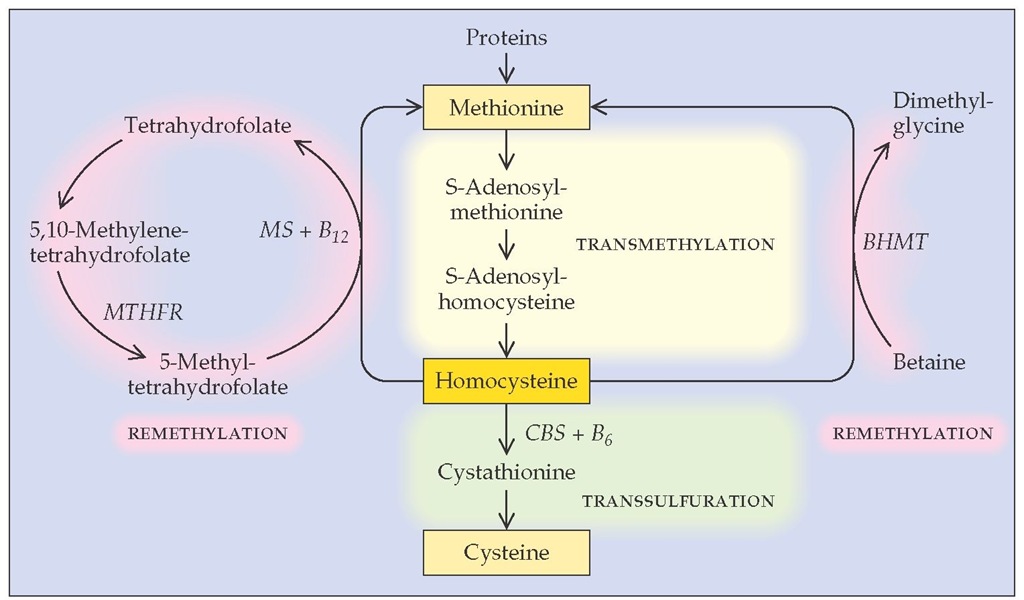
Homocysteine is a highly reactive amino acid that is normally found in blood at levels of 5 to 15 mmol/L. Normally, homocys-teine is derived from methionine by a transmethylation process and is remethylated to methionine or converted to cysteine [see Figure 2]. Metabolism of homocysteine requires betaine, cobal-amin (vitamin B12), folate, and pyridoxine (vitamin B6).
Homocysteine can promote oxidation of low-density lipopro-tein (LDL) cholesterol and presumably is toxic to vascular en-dothelium.43,44 It may also inhibit thrombomodulin expression and protein C activation and suppress endothelial heparan sul-fate expression; both of these effects lead to hypercoagulabili-ty.45,46 Homocysteine also enhances the binding of lipoprotein(a) (an atherogenic lipoprotein) to fibrin, which may provide a link between hyperhomocysteinemia, thrombosis, and premature atherosclerosis [see Lipoprotein(a), below].47 The vascular damage caused by high homocysteine levels leads to arterial and venous thrombosis and, perhaps, accelerated atherosclerosis.
Epidemiology and Etiology
Hyperhomocysteinemia can be divided into three classes: severe (homocysteine plasma concentration > 100 mmol/L), moderate (25 to 100 mmol/L), or mild (16 to 24 mmol/L). Severe hy-perhomocysteinemia is usually caused by a homozygous deficiency of the enzyme cystathionine ^-synthase. Affected persons have severe mental retardation, ectopic lens, skeletal abnormalities, and severe early-onset arterial and venous thrombotic disease.48
Mild or moderate hyperhomocysteinemia results from either hereditary or acquired defects in the homocysteine metabolic pathway. Heterozygous deficiency in cystathionine ^-synthase is quite common in the general population, with a frequency of 0.3% to 1.4%.® A defect in the remethylation pathway is commonly caused by a thermolabile mutant of the methylene-tetrahydrofolate reductase (MTHFR) enzyme whose activity is approximately 50% of normal; the homozygous state has a prevalence of 5% in the general population.49 However, the ho-mozygous form of the MTHFR thermolabile enzyme isoform is not clinically relevant in patients whose diet includes adequate folate.
Common causes of acquired hyperhomocysteinemia are deficiencies of dietary cobalamin, folate, or pyridoxine. A prospective study found that mild hyperhomocysteinemia is quite common in the elderly, despite normal serum vitamin concentra-tions.50 Acquired hyperhomocysteinemia is also common in patients with end-stage renal disease.
Mild to moderate hyperhomocysteinemia is associated with cerebrovascular disease, coronary artery disease, and peripheral vascular disease in persons younger than 55 years and with carotid artery stenosis in the elderly.51,52 It is found in 10% of patients with a first episode of DVT.53 In a prospective study, a graded relationship was found between elevated plasma homo-cysteine levels and mortality in patients with coronary artery disease.54
Clinical Presentation
Severe hyperhomocysteinemia should be suspected in patients with the characteristic phenotype (see above). Mild to moderate hyperhomocysteinemia should be suspected in cases of arterial and venous thrombotic disease—including cere-brovascular disease, peripheral arterial disease, and DVT—es-pecially in young persons.
Diagnosis
Plasma homocysteine exists in free and protein-bound forms and is generally measured and reported as total plasma homo-cysteine (normal range, 5 to 15 mmol/L). Diagnosis of hyperho-mocysteinemia is usually made by measuring plasma homocys-teine levels after an overnight fast. Because as many as 40% of patients with hyperhomocysteinemia may have a normal fasting level, a methionine-loading test should be considered when indicated.55 However, methionine is not generally available in most pharmacies. Plasma folate and vitamin B12 levels should also be measured to exclude hyperhomocysteinemia caused by folate or B12 deficiencies.
Figure 2 Homocysteine’s intracellular metabolism occurs through remethylation to methionine or transsulfuration to cysteine.142 Elevation in plasma homocysteine levels can result from hereditary deficiency in cystathionine p-synthase (CBS); a thermolabile mutant of methylene-tetrahydrofolate reductase (MTHFR); or low dietary levels of cobalamin (vitamin B^), folate, or pyridoxine (vitamin B6), which are essential cofactors in the metabolic process. (BHMT—betaine-homocysteine methyltransferase)
Treatment
Daily use of oral pyridoxine (250 mg) and folic acid (5 mg) brings elevated homocysteine levels down to normal in most cases.56 Patients who have vitamin B12 deficiency should be given B12 supplements. Repeat measurement of plasma homocysteine levels (generally done 1 month after starting supplementation) may be prudent to ensure that the treatment with pyridoxine and fo-late is working. Betaine (3 g p.o., b.i.d.) is sometimes effective in patients with hyperhomocysteinemia that is resistant to pyridox-ine and folate. It is currently unknown whether correction of hy-perhomocysteinemia by these measures leads to clinical benefit.
Lipoprotein (A)
Lipoprotein(a) [Lp(a)] is an independent risk factor for coronary artery thrombosis.57 A prospective case-control study associated elevated plasma Lp(a) levels with an approximately threefold increase in risk of coronary artery disease in men.58 The association between high Lp(a) and ischemic stroke in young adults is controversial.59,60 Distributions of Lp(a) are skewed in the general population—especially among whites, in whom the median is 3.7 mg/dl but the mean is 6.9 mg/dl.61 The 95th percentile for plasma Lp(a) is estimated to be in the 25 to 30 mg/dl range.
The Lp(a) class of lipoproteins is formed by the assembly of LDL particles and apoprotein(a), a protein that has some structural similarities to plasminogen (specifically, in the kringle domains) and competes with plasminogen for the endothelial cell binding site, thereby displacing plasminogen and downregulat-ing plasmin generation at the endothelial cell surface.62 High plasma concentrations of Lp(a) may therefore suppress the en-dothelial fibrinolytic response. Lp(a) is found in the intima of human atherosclerotic vessels, and transgenic mice expressing human Lp(a) develop extensive atherosclerosis.63 Measurement of Lp(a) levels can be done in commercial laboratories and should be considered in young patients with arterial thrombosis. Elevated LDL cholesterol levels appear to elicit or exacerbate the risk factors associated with high Lp(a), and therefore, diet,exercise, and standard pharmacologic approaches should be used in patients with high LDL cholesterol levels.64 In small studies, niacin at high doses (2 to 4 g p.o. daily) and tamoxifen (20 mg daily) have lowered elevated Lp(a) levels by 30% to 40%.65,66 High doses of niacin are frequently associated with facial flushing and headaches. These unpleasant side effects can be ameliorated by starting niacin at a low dose (e.g., 300 mg daily) and then increasing the dose incrementally over time or through the use of extended-release niacin. Liver function should be checked periodically.
Dysfibrinogenemia
Approximately 300 abnormal fibrinogens (dysfibrinogens) have been reported, and about 85 structural defects have been identified in dysfibrinogenemia. These are most commonly characterized by functional defects of fibrinopeptide A release and fibrin polymerization and less commonly by defective plas-minogen binding and activation. About half of the fibrinogen mutations are not associated with any clinical symptoms. Mild bleeding or recurrent thrombosis occurs in about equal numbers in the remaining mutations.67 In rare cases, patients experience both bleeding and thrombosis. Acquired dysfibrinogenemia may complicate hepatocellular carcinoma or chronic liver disease. Evaluation in a general laboratory usually shows a discrepancy between antigenic and functional levels of fibrinogen, because most patients with dysfibrinogens have suboptimal clotting function, with prolonged thrombin time (TT) and repti-lase time (RT). The abnormal fibrinogens form fibrin clots that are resistant to clot lysis. Precise identification of the structural defect requires substantial effort in a research laboratory. Management of recurrent thrombosis caused by dysfibrinogenemia is the same as that in other patients with thrombophilia.