Restoring arterial oxygenation
An increase in the FjO2 may suffice to restore normal oxygenation. On the basis of the shape of the oxygen-hemoglobin dissociation curve, achieving a Pao2 of 60 mm Hg or higher (> 90% oxygen saturation of hemoglobin) is an optimal goal for most acutely hypoxemic patients. In patients with chronic hypoxemia and hypercapnia, a Pao2 of 50 to 55 mm Hg may be needed to prevent respiratory depression and worsening hypercapnia.
Supplemental oxygen can be administered by a number of techniques to the spontaneously breathing patient. Nasal can-nulas allow patients to eat, drink, and speak during oxygen administration. Their disadvantage is that the exact Fio2 delivered is not known, because it is influenced by the patient’s peak inspiratory flow demand. As an approximation, the following guide can be used: 1 L/min of nasal-prong oxygen flow is approximately equivalent to an Fio2 of 24%, with each additional 1 L of flow increasing the Fio2 by approximately 4%. Flow rates should be limited to less than 5 L/min. Venturi masks allow more precise administration of oxygen to be delivered, with calibrated mask Fio2 values between 24% and 50%. Often, Venturi masks are useful in patients with COPD and hypercapnia because one can titrate the Pao2 to minimize carbon dioxide retention. Nonrebreathing masks achieve higher oxygen concentrations (approximately 80% to 90%) than partial rebreathing systems. A one-way valve prevents exhaled gases from entering the reservoir bag in a nonrebreathing system, thereby maximizing the Fio2.
A continuous positive airway pressure (CPAP) mask can be used if the Pao2 is less than 60 to 65 mm Hg during use of a non-rebreathing mask and the patient is conscious and cooperative, able to protect the lower airway, and hemodynamically stable. CPAP is delivered by a tight-fitting mask equipped with pressure-limiting valves. Many patients cannot tolerate a CPAP mask because of persistent hypoxemia, hemodynamic instability, or feelings of claustrophobia or aerophagia. In such patients, endotracheal intubation should be performed. Initially, 3 to 5 cm H20 of CPAP should be applied while monitoring the Pao2 or arterial oxygen saturation (Sao2). If the Pao2 is still less than 60 mm Hg (Sao2 < 90%), the level of CPAP should be increased in increments of 3 to 5 cm H20 up to a level of 10 to 15 cm H20. CPAP may improve oxygenation by opening previously closed alveoli and decreasing intrapulmonary shunting. Although the use of CPAP may prevent the need for intubation and mechanical ventilation, it may result in such complications as gastric distention, drying of the eyes from air leaks, and skin breakdown, especially on the bridge of the nose.4 Bilevel positive airway pressure (BiPAP) is a method of noninvasive ventilation in which inspiratory and expiratory pressure can be applied through a mask during the patient’s respiratory cycle. The inspiratory support decreases the patient’s work of breathing. The expiratory support (CPAP) improves gas exchange by preventing alveolar collapse. Noninvasive ventilation using face or nasal masks may obviate endotracheal intubation and mechanical ventilation in patients with neuromuscular disease, COPD, and postoperative respiratory insufficiency.
In using BiPAP, a pressure-support ventilation (PSV) level of 5 to 10 cm H2O and a CPAP level of 3 to 5 cm H2O are reasonable starting points. The PSV level can be increased in increments of 3 to 5 cm H2O, using the patient’s respiratory rate as a guide of effectiveness.
Mechanical ventilatory support
When adequate oxygenation cannot be maintained by nonin-vasive means or if progressive hypoventilation and hypercapnia with respiratory acidosis occurs, endotracheal intubation and mechanical ventilatory support should be initiated. Mechanical ventilation can produce positive pressure at the airway opening or create negative pressure around the chest wall. Use of negative pressure ventilators (e.g., an iron lung) is generally restricted to patients with chronic neuromuscular weakness or chest wall deformity.
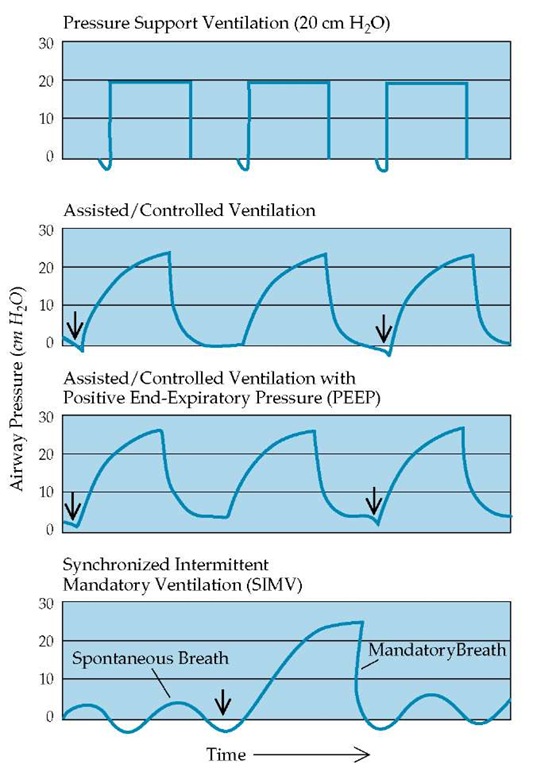
Figure 2 Pressures measured at the endotracheal tube are shown as a function of time to illustrate the effects of various modes of pressure-preset (top panel) or constant tidal volume (bottom three panels) mechanical ventilation. Supra-atmospheric pressures have positive values, whereas subatmospheric pressures have negative values. Arrows indicate initiation of inspiration by the patient, triggering the ventilator to deliver an assisted breath.
Volume-Cycled and Pressure-Cycled Ventilation
There are two basic types of positive pressure ventilation: volume cycled and pressure cycled.5,6 Volume-cycled ventilation, the more commonly used mode in adults, supplies a fixed tidal volume, making inflation pressure the dependent variable. When a volume-cycled ventilator is used, changes occurring in pulmonary impedance are associated with alterations in the airway pressures during inflation. A pressure limit can be set using a pop-off valve that prevents further inflation, avoiding excessive overdistention, and functions as an alarm.
Pressure-cycled ventilation provides gas flow until a preset pressure is reached, so that the tidal volume becomes the dependent variable. With this mode, changes in pulmonary impedance are associated with alterations in tidal volume and therefore minute ventilation.
With volume-cycled ventilation, two primary modes are used: assisted/controlled (A/C) ventilation and synchronized intermittent mandatory ventilation (SIMV) [see Figure 2]. In the A/C mode, the ventilator guarantees a preset number of breaths (backup rate) supplied at a preset tidal volume. If the patient desires to breathe at a respiratory rate higher than the preset rate, the ventilator will deliver the entire preset tidal volume every time the patient generates a small negative airway pressure (-1 to -2 cm H2O). In the SIMV mode, the ventilator also guarantees a preset number of breaths (backup rate) supplied at a preset tidal volume. However, the tidal volume for any additional breath above the preset rate is determined by the effort of the patient. Therefore, the SIMV mode combines a preset number of ventilator-delivered mandatory breaths with the ability to assist intermittent patient-generated spontaneous breaths. Most patients can be effectively ventilated with either A/C or SIMV, and both modes can be used with pressure-controlled ventilators. The A/C mode has certain advantages over SIMV in that the former usually requires less respiratory effort, reduces oxygen consumption, and is more likely to rest respiratory muscles. However, the respiratory muscles may continue to perform considerable work during A/C breathing, especially when respiratory drive and minute ventilation are increased. Potential advantages of SIMV include the exercising of respiratory muscles, the prevention of respiratory alkalemia, and, possibly, improved patient-ventilator coordination. However, a change from A/C ventilation to SIMV does not usually correct respiratory alkalemia caused by an increased respiratory drive.
Pressure-Support Ventilation
PSV is a pressure-targeted, flow-cycled mode that requires the patient to initiate every breath [see Figure 2]. During the inspirato-ry phase, pressures rise rapidly to a preset plateau level. The pressure terminates when the inspiratory airflow created by the patient falls below a certain level. Therefore, the total work of breathing for each breath is usually generated by a combination of patient effort and mechanical support. Intubated patients can be ventilated with pressure support alone, as long as they have an adequate ventilatory drive and are strong enough to initiate a sufficient number of breaths to maintain adequate minute ventilation. When used as the primary mode of ventilation, pressure support can be used with certain amounts of CPAP and generally is well tolerated by patients who are being weaned from mechanical ventilation. The pressure support mode of ventilation can also be used in conjunction with SIMV, in which case the level of pressure support is applied only to patient-generated breaths.
Selecting Appropriate Mechanical Ventilatory Settings
After the patient is intubated, the respiratory rate and tidal volume (in volume-controlled ventilation) should be set to maintain an adequate minute ventilation that will result in an appropriate pH level. The tidal volume should be large enough to prevent microatelectasis and progressive hypoxemia but without causing barotrauma. For most patients, an initial tidal volume between 6 and 10 ml/kg body weight is appropriate [see Management of Respiratory Failure in Specific Clinical Settings, Acute Respiratory Distress Syndrome, below].
Positive end-expiratory pressure (PEEP) is used to improve oxygenation and reduce the possibility of oxygen toxicity for patients requiring high levels of inspired oxygen. PEEP works by opening or recruiting previously closed alveoli and redistributing lung water from the alveoli to the interstitial spaces. CPAP works in a similar manner for patients who are breathing spontaneously. The benefits of PEEP must be weighed against its deleterious effects, which include decreased cardiac output, increased intracranial pressure, and hyperinflation.
Selecting the appropriate mode, respiratory rate, tidal volume (or pressure, in pressure-controlled ventilation), and amount of PEEP is a dynamic process. No single setting is appropriate for all patients or for a given patient at all stages of acute illness. Optimal ventilatory settings require constant monitoring and numerous readjustments based on acute changes in gas exchange, airway pressures, breathing patterns, and hemodynamics in conjunction with the resolution or progression of the underlying disease process.
During volume-cycled ventilation, peak and plateau airway pressures can be easily seen from most ventilators. Peak airway pressure can be subdivided into three components: (1) flow resistive, (2) elastic distending pressure, and (3) PEEP (either set or intrinsic) [see Figure 3]. The plateau pressure is a close approximation of the alveolar pressure and can be subdivided into only two components: (1) elastic distending pressure and (2) PEEP (either set or intrinsic). Because positive pressure is required to overcome the flow-resistive properties of the airway and external apparatus, the peak pressure will always be equal to or greater than the plateau pressure. The plateau pressure is measured by interrupting flow or pausing the ventilator at full inspiration. The static compliance of the respiratory system is computed by dividing the tidal volume delivered to the patient by the change in pressure (plateau pressure minus PEEP). A reduction in the normal static compliance is indicative of a stiffer lung or chest wall.
Figure 3 In volume-cycled pressure ventilation, the total pressure required to overcome flow-resistive and elastic forces and deliver the preset tidal volume is termed peak airway pressure. The plateau pressure is the pressure required to keep the lung and chest wall inflated with the preset tidal volume during a brief period in which there is no flow (inspiratory pause). The difference between the peak and the plateau pressures is a reflection of the flow-resistive component of ventilation and is increased when airway resistance or inspiratory flow rate is increased. The plateau pressure is a function of lung and chest wall compliance and is unaffected by airway resistance and inspiratory flow rate unless auto-PEEP (positive end-expiratory pressure) occurs. Incomplete emptying is associated with auto-PEEP, the magnitude of which can be assessed with end-expiratory airway occlusion.
During volume-cycled ventilation, changes in airway pressures can be helpful in determining the cause of an acute deterioration in mechanically ventilated patients. A large increase in peak airway pressure (out of proportion to the increase in plateau pressure) signals a change in the flow-resistive properties. This increase is usually observed in patients with airway problems (e.g., kinking of the endotracheal tube, mucous plug, or bronchospasm). Treatment should be directed at improving airway function, including repositioning the endotracheal tube, suctioning the airways, and administering bronchodilator therapy. When an increase is primarily in plateau pressure, the problem is localized to the lung parenchyma or chest wall rather than the airways. This condition can be seen with worsening of pulmonary edema, tension pneumothorax, a large region of atelectasis, or intubation of the right mainstem bronchus.
With both positive and negative pressure ventilators, exhalation is passive and is provided by the elastic recoil of the inflated lungs and chest wall. Exhalation rate depends on the resistance and compliance of the respiratory system. A high resistance is observed in patients who require a prolonged exhalation time, such as those with chronic airway obstruction or asthma. If the subsequent tidal volume is delivered before exhalation is complete, positive airway pressure will be maintained throughout the respiratory cycle, resulting in increases in end-expiratory lung volume and end-expiratory alveolar pressure. PEEP that results from this process of dynamic hyperinflation has been termed auto-PEEP (or intrinsic PEEP). Auto-PEEP increases both peak and plateau pressures and can be measured by briefly occluding the airway at the end of exhalation.7 When the airflow is interrupted, pressures equilibrate quickly, and the airway-opening pressure rises to the level of the previous alveolar pressure, which is the level of auto-PEEP. The appropriate compliance of the respiratory system can be calculated by dividing the tidal volume by the difference between the plateau pressure and the auto-PEEP.
Management of Respiratory Failure in Specific Clinical Settings
Chronic obstructive pulmonary disease
Patients with severe COPD are often hypoxemic and hyper-capnic on a long-term basis and adapt, albeit precariously, to their abnormal state. Acute deterioration is most often triggered by infection, but it may also result from factors such as pneu-mothorax, congestive heart failure, and increased C02 production associated with febrile states. The worsening hypoxemia and hypercapnia that accompany acute deterioration lead to increasing dyspnea, sleep disruption, and, occasionally, alterations in consciousness. Depressed consciousness leads to retention of secretions and further worsening of gas exchange. This cycle can be broken by identifying and rectifying the processes that have precipitated the acute deterioration and by providing support to improve gas exchange while the underlying disorders are being corrected.
Arterial Blood Gas Analysis
Arterial blood gas analysis is crucial to the proper assessment and management of acute exacerbations. The first priority is to achieve a PaO2 level of 50 to 60 mm Hg but no higher. An elevation in PaCO2 of 10 to 15 mm Hg is common when oxygen is given to patients with chronic airway obstruction and does not represent a failure of controlled oxygen therapy, provided that there is no critical reduction in blood pH (i.e., < 7.2). Therapy should include promotion of bronchopulmonary drainage by encouragement of cough, administration of inhaled broncho-dilators and systemic corticosteroids, and treatment of any underlying infection.8
Ventilatory Support
Many patients with severe exacerbations of COPD experience persistent respiratory acidosis and excessive work in breathing even after their initial treatment with bronchodilators. The level for PaCO2 at which ventilatory assistance becomes necessary cannot be specified, but ventilatory supports should be considered if hypercapnia is severe enough to cause profound acidemia (pH < 7.2) or if the patient shows signs of altered mental status or respiratory muscle fatigue. Intubation should be performed if hemodynamic instability or somnolence occurs or if secretions cannot be cleared. However, if the hypercapnic patient remains alert and cooperative, delivery of noninvasive positive pressure ventilation through a facial or nasal mask may reverse or prevent fatigue and thereby eliminate the need for conventional mechanical ventilation through an endotracheal tube. In selected patients with severe exacerbations of COPD, noninvasive ventilation decreases the rate of complications, reduces the need for intubation, shortens hospital stay, and may lower mortali-ty.9,10 Patients who do not tolerate the mask or whose acute exacerbation fails to improve should be intubated. The delay in intubation incurred by an unsuccessful trial of noninvasive ventilation should not pose a significant risk to the patient, provided that personnel skilled in airway management and intubation are readily available and the patient does not have hemodynamic instability or significant underlying cardiac disease. The success rate for noninvasive ventilation is highly dependent on the skill and commitment of the respiratory therapists and nurses who work with the patient.
Noninvasive ventilation The most common method of noninvasive ventilation for patients with airway obstruction is BiPAP. This type of ventilation delivers a specified amount of in-spiratory positive airway pressure (IPAP), usually in the form of PSV, that supports each spontaneous breath (usually set at 15 to 20 cm H2O) in conjunction with a low level of expiratory positive airway pressure (EPAP) (usually set at 3 to 5 cm H2O). Some patients with acute hypercapnic respiratory failure tolerate a full face mask better than a nose mask because of large leaks that occur with the nose mask. However, securing the mask so tightly that all leaks are prevented may increase discomfort and decrease the ultimate likelihood of success.
Invasive ventilation For exacerbations that require intubation, a reasonable initial ventilator setting is a tidal volume of 8 to 10 ml/kg at 11 to 14 breaths a minute.11 It is important to remember that PaCO2 levels in patients with chronic hypercapnia should not be lowered to the normal range, because this could result in alkalemia, which increases the risk of cardiac dysrhythmias and seizures. In addition, overventilation for more than 2 to 3 days may result in renal restoration of the pH to normal. As a consequence, during subsequent trials of spontaneous ventilation, as the PaCO2 rises to the baseline hypercapnic level, the patient becomes acidemic or the patient’s respiratory muscles become fatigued because of the greater minute ventilation required for the reset baseline pH and PaCO2. To prevent this condition, adjustments in respiratory frequency and tidal volume should be aimed at maintaining the patient’s baseline pH and PaCO2 or allowing some further degree of tolerated respiratory acidosis.
Dynamic hyperinflation with auto-PEEP occurs frequently during mechanical ventilation of patients with airflow obstruction [see Mechanical Ventilatory Support, above]. For airflow obstruction, the most critical determinant of the severity of dynamic hyperinflation is the minute ventilation delivered by the mechanical ventilator. Therefore, in addition to the effects on PaCO2 and pH levels, excessive ventilation of patients with airflow obstruction increases the risk of pulmonary hyperinflation. The major consequences of excessive auto-PEEP include barotrau-ma, misinterpretation of central venous and pulmonary arterial wedge pressures, decreased cardiac output secondary to reduced venous return, and increased work in breathing. The effort required to breathe increases because the patient’s inspirato-ry muscles must generate a negative pressure equal to the auto-PEEP before the proximal airway pressure can be lowered to the -1 to -2 cm H2O required to trigger the ventilator. Auto-PEEP should be suspected in a patient with airflow obstruction when the ventilator does not deliver an assisted breath even though the patient is making obvious inspiratory efforts. Inspiratory effort can be aided by the application of external PEEP at a level equal to or slightly less than the auto-PEEP level. Applying external PEEP causes the ventilator to deliver an assisted breath when the proximal airway pressure is lowered to 1 to 2 cm H2O below the level of applied PEEP rather than to -1 to -2 cm H2O. In effect, the application of external PEEP reduces the inspirato-ry effort required to trigger the ventilator. In patients with chronic airflow obstruction, applied PEEP does not increase lung volume or airway pressures as long as the level of applied PEEP does not exceed the level of auto-PEEP.12
Asthma
Respiratory failure in children and adults with asthma is usually precipitated by the underlying disease itself, viral or bacterial infection, irritation of the airway from aspiration or inhalation of a toxic gas, or medical noncompliance. Asthmatics usually have a mixed form of respiratory failure with both hypoxemia and hypercapnia.
Ventilatory Support
The goals of ventilatory support in a patient with asthma are to reverse the hypoxemia by administering supplemental oxygen and to reverse the hypercapnia and respiratory acidosis (assuming the patient is not a chronic retainer of CO2). The approach to ventilatory support is similar to that outlined above for patients with acute exacerbations of COPD. However, patients with asthma may have a greater degree of airway obstruction, which may necessitate additional modes of therapy. The use of inhaled mixtures of helium and oxygen (usually composed of at least 70% helium) can reduce the density of the inspired gases, improving overall ventilation and reducing the patient’s work associated with ventilation. However, in patients requiring increasing supplemental oxygen because of concomitant hypoxemia, the beneficial effects of the helium-oxygen mixture (heliox) may be lost.
Acute respiratory distress syndrome
Hypoxemic respiratory failure in patients with ARDS results in an unacceptably high mortality (30% to 60%). It may occur in previously healthy patients without preexisting lung disease. The pathophysiology, clinical features, and nonventilatory therapies for ARDS are outlined elsewhere13 [see 14:X Pulmonary Edema].
Ventilatory Support
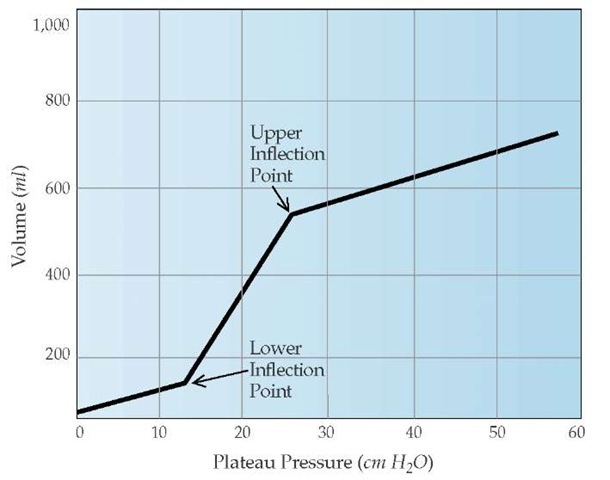
The initiation of mechanical ventilation for ARDS patients clearly has beneficial effects, yet a growing body of evidence indicates that mechanical ventilation can produce pathologic changes in normal lung tissue that are similar to ARDS. Experimentally, large inflation pressures produce capillary leak and, over time, permeability pulmonary edema. Harmful effects of large inflations and subsequent high alveolar pressures have been termed volutrauma.14 Mechanical ventilation strategies for ARDS patients have been supported by studies that examined computed tomography scans and pressure-volume curves. Although chest radiographs in ARDS patients suggest homogeneous involvement of the lungs, CT scans reveal a markedly heterogeneous pattern.15 Dependent regions of the lung are consolidated, but more superior regions of the lung appear normal. The inflation portion of the pressure-volume curve of ARDS patients exhibits a lower inflection point, which corresponds to the onset of reopening closed alveoli, and an upper inflection point, which signals the beginning of the overdistention of patent alveoli16 [see Figure 4].
A specific ventilatory strategy that focuses on ventilating patients between the upper and lower inflection points has been proposed for ARDS patients. To limit transpulmonary pressures (plateau pressure of < 30 cm H20), low tidal volumes should be used. The subsequent decrease in minute ventilation, which is caused by the use of low tidal volumes and may result in hyper-capnia and respiratory acidosis, is termed permissive hypercap-nia. This strategy of mechanical ventilation with lower tidal volumes (6 ml/kg) versus traditional ventilation with larger tidal volumes (12 ml/kg) results in decreased mortality and shortens the amount of time that mechanical ventilation is required.17
Use of PEEP
PEEP is an effective way to improve oxygenation. However, the optimal amount of PEEP that should be used is controversial. The titration of PEEP to a level above the lower inflection point may decrease the damage to alveoli that would be caused by repetitive reopening and closing of lung units. The application of this level of PEEP, called the open lung approach, was associated with improved mortality when compared with conventional ventilatory strategies.18 The beneficial effects of PEEP must always be weighed against a possible decrease in cardiac output and increased risk of barotrauma.
Inverse-Ratio Ventilation
One strategy for improving oxygenation without increasing FjO2 or PEEP is to prolong inspiratory time by adding an end-in-spiratory pause, keeping alveolar pressure briefly at the plateau level.19 When inspiratory time is prolonged, exceeding expiratory time, the ventilation is termed inverse-ratio ventilation. Inverse-ratio ventilation may improve oxygenation in some ARDS patients; however, prospective studies have found that most patients do not benefit from it.20 Caution is required in applying inverse-ratio ventilation because barotrauma and hypotension can result from the development of excessive auto-PEEP as expiratory time is shortened.
Figure 4 A static pressure-volume curve can be constructed by plotting the tidal volume versus the elastic recoil pressure of the respiratory system for several different tidal volumes. In patients with acute respiratory distress syndrome, the inflation curve demonstrates a lower inflection point, which corresponds to the onset of reopening closed alveoli, and an upper inflection point, which signals the beginning of the overdistention of patent alveoli.