The major physiologic function of the lungs is gas exchange between the pulmonary capillaries and the alveolar space. Oxygen is taken up from the alveolar space onto hemoglobin in red blood cells for transport to peripheral tissues; carbon dioxide is eliminated after it is carried to the lungs via the venous circulation. Acute respiratory failure is the inability of the respiratory system to provide adequate gas exchange. This can take the form of inadequate oxygenation of the arterial blood, insufficient removal of carbon dioxide from venous blood, or both. Acute respiratory failure can be caused by lesions affecting several parts of the respiratory system, including the airways, lung parenchyma, chest wall and respiratory muscles, and neuro-muscular processes involved in breathing [see Table 1].
Definitions and Pathogenesis
Acute hypoxemic respiratory failure
Definition
Acute hypoxemic respiratory failure is defined as a decrease in the delivery of oxygen from the atmosphere to the blood and, more specifically, as an arterial oxygen tension (PaO2) of less than 60 mm Hg. Although somewhat subjective, this value was selected on the basis of the beginning of the descent of the oxygen-hemoglobin dissociation curve at a PO2 of 60 mm Hg.
Pathogenesis
The causes of acute hypoxemic respiratory failure are a low inspired concentration of oxygen, impairment of oxygen diffusion, alveolar hypoventilation, ventilation-perfusion (V/Q) mismatch, intrapulmonary shunting, and a low mixed venous oxygen content.
Low inspired concentration of oxygen is an uncommon cause of acute hypoxemic respiratory failure. This can occur at high altitudes or when toxic gases are inhaled (e.g., smoke inhalation). In patients with other cardiopulmonary disease processes, an inappropriately low fraction of inspired oxygen (FIO2) can contribute to hypoxic respiratory failure. Acute hypoxemic respiratory failure resulting from diffusion impairment is also relatively uncommon. This usually occurs in the setting of acute or chronic interstitial lung disease. Patients with acute respiratory distress syndrome (ARDS) can also have diffusion impairments contributing to hypoxemia, but shunting is the more important physiologic derangement in this disorder (see below). Both low inspired concentrations of oxygen and diffusion impairments usually respond to supplemental oxygen therapy.
Pure alveolar hypoventilation is a relatively rare form of acute hypoxemic respiratory failure that is caused by neuromus-cular or central nervous system dysfunction (e.g., opiate overdose). The lung parenchyma is essentially normal. This condition is characterized by an acute reduction in effective alveolar ventilation and a subsequent decrease in the amount of CO2 that is eliminated by the lungs. Therefore, the arterial carbon dioxide tension (PaCO2) and the PaO2 are always increased in patients with pure alveolar hypoventilation. Hypoxemia occurs as a result of the displacement of oxygen in the alveolar space resulting from the failure to eliminate CO2. Calculation of the alveolar-arterial oxygen gradient or difference [A-aDO2] is helpful in determining whether acute hypoxemic respiratory failure is from pure alveolar hypoventilation. With pure alveolar hypoventila-tion, the A-aDO2 is in the normal range of 5 to 25 mm Hg when the patient is breathing room air. However, when acute hypox-emic respiratory failure is caused by![]() mismatching or an in-trapulmonary shunt, the A-aDO2 is always increased.
mismatching or an in-trapulmonary shunt, the A-aDO2 is always increased.
![]() mismatching is the most common pathophysiologic cause of acute hypoxemia. It develops when there is a decrease in ventilation to normally perfused regions of the lung, a decrease in perfusion to normally ventilated regions of the lung, or some combination of a decrease in both ventilation and perfu-sion. Regions of the lung with low
mismatching is the most common pathophysiologic cause of acute hypoxemia. It develops when there is a decrease in ventilation to normally perfused regions of the lung, a decrease in perfusion to normally ventilated regions of the lung, or some combination of a decrease in both ventilation and perfu-sion. Regions of the lung with low![]() ratios caused by inadequate ventilation primarily result in arterial hypoxemia. By contrast, regions of the lung with high
ratios caused by inadequate ventilation primarily result in arterial hypoxemia. By contrast, regions of the lung with high![]() ratios caused by inadequate perfusion result in wasted ventilation, or dead-space ventilation, and are typically associated with hypercapnia when severe. The degree of hypoxemia in patients with pure
ratios caused by inadequate perfusion result in wasted ventilation, or dead-space ventilation, and are typically associated with hypercapnia when severe. The degree of hypoxemia in patients with pure![]() mismatching improves in response to an increase in the
mismatching improves in response to an increase in the![]() This correction occurs because airways to poorly ventilated alveoli remain patent, and the increased inspired oxygen will eventually reach pulmonary capillary blood. Another clinical use for the calculation of the
This correction occurs because airways to poorly ventilated alveoli remain patent, and the increased inspired oxygen will eventually reach pulmonary capillary blood. Another clinical use for the calculation of the![]() is to identify
is to identify![]() mismatching, when the measured PaO2 is normalized by hyperventilation. For example, a PaO2 of 90 mm Hg and a PaCO2 of 20 mm Hg when breathing room air would represent significant
mismatching, when the measured PaO2 is normalized by hyperventilation. For example, a PaO2 of 90 mm Hg and a PaCO2 of 20 mm Hg when breathing room air would represent significant![]() mismatching, as evidenced by the calculated .
mismatching, as evidenced by the calculated .![]() of 35 mm Hg.
of 35 mm Hg.
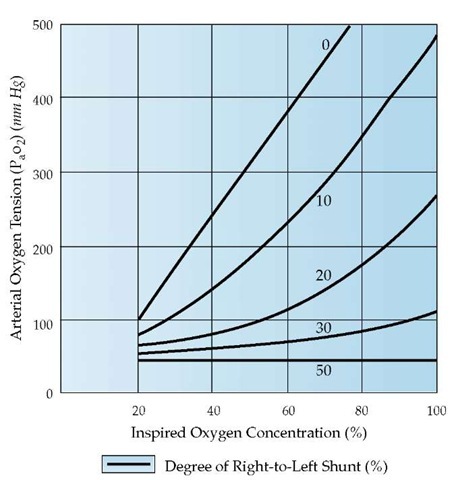
Physiologic shunting occurs when venous blood bypasses ventilated alveoli and enters the arterial system. In the normal lung, a 2% to 3% shunt normally occurs because of the bronchial artery circulation and drainage of some coronary venous blood directly into the left ventricle via the thebesian veins. In patients with severe pneumonia, atelectasis, or pulmonary edema, intra-pulmonary shunting occurs when pulmonary capillary blood passes next to alveoli that are completely collapsed or filled with edema fluid or inflammatory cells. Shunting can be differentiated from![]() mismatching on the basis of the differences in the response to inhalation of 100% oxygen. PaO2 levels in patients with shunting who receive 100% oxygen will not improve to normal. In fact, the change in the PaO2 level that occurs in response to FIO2 values allows the shunt to be estimated as a percentage of cardiac output, assuming normal values for the difference in oxygen content between arterial and mixed venous blood and
mismatching on the basis of the differences in the response to inhalation of 100% oxygen. PaO2 levels in patients with shunting who receive 100% oxygen will not improve to normal. In fact, the change in the PaO2 level that occurs in response to FIO2 values allows the shunt to be estimated as a percentage of cardiac output, assuming normal values for the difference in oxygen content between arterial and mixed venous blood and![]() [see Figure 1]. With a shunt of 30% or greater, the
[see Figure 1]. With a shunt of 30% or greater, the![]() rises little, if at all, with increasing FIO2. By contrast, the rise in P O2 with oxygen therapy is appreciable even with a severe
rises little, if at all, with increasing FIO2. By contrast, the rise in P O2 with oxygen therapy is appreciable even with a severe![]() mismatch.
mismatch.
Low mixed venous oxygenation can also contribute to hypox-emia. However, it is uncommon for this to be the only factor contributing to acute hypoxemic respiratory failure. Normally, the lungs fully oxygenate pulmonary arterial blood, and mixed venous oxygen tension (P-vo2) does not affect![]() significantly. However, a decreased P-vo2 can lower the Pao2 significantly when either intrapulmonary shunting or
significantly. However, a decreased P-vo2 can lower the Pao2 significantly when either intrapulmonary shunting or![]() mismatch is present. Factors that can contribute to low mixed venous oxygena-tion include anemia, hypoxemia, inadequate cardiac output (CO) as occurs in cardiogenic shock, and increased oxygen consumption. Improving oxygen delivery to tissues by increasing hemoglobin or CO usually decreases oxygen extraction and improves P-vo2 and, subsequently, Pao2.
mismatch is present. Factors that can contribute to low mixed venous oxygena-tion include anemia, hypoxemia, inadequate cardiac output (CO) as occurs in cardiogenic shock, and increased oxygen consumption. Improving oxygen delivery to tissues by increasing hemoglobin or CO usually decreases oxygen extraction and improves P-vo2 and, subsequently, Pao2.
Table 1 Causes of Acute Respiratory Failure
|
Acute Hypoxemic Respiratory Failure |
Acute Hypercapnic Respiratory Failure |
Mixed Respiratory Failure |
|
Lung parenchymal disease process |
Lung parenchymal disease process |
Includes many disorders that can be associated with both hypoxemia and hypercapnia |
|
Pneumonia |
Emphysema |
|
|
Aspiration |
Interstitial lung disease (usually end stage) |
|
|
Acute lung injury/acute respiratory distress |
Airway disease process (upper and lower airways) |
|
|
syndrome |
Asthma |
|
|
Asthma |
obstructive sleep apnea |
|
|
Emphysema/chronic bronchitis |
Vocal cord dysfunction |
|
|
Smoke inhalation |
Airway obstruction |
|
|
Interstitial lung disease |
Chest wall/pleural space disease process |
|
|
Atelectasis |
obesity hypoventilation syndrome |
|
|
Radiation injury |
Kyphoscoliosis |
|
|
Cardiogenic pulmonary edema |
Pleural effusion |
|
|
Pulmonary vascular disease process |
Pleural fibrosis |
|
|
Pulmonary embolism |
Traumatic flail chest |
|
|
Fat embolism |
Pneumothorax |
|
|
Air embolism |
Neuromuscular disease process |
|
|
Airway disease process |
Brain |
|
|
Airway obstruction |
Narcotic overdose |
|
|
Asthma |
Cerebrovascular accident |
|
|
Airway edema (heat injury, allergic reaction) |
Encephalitis/meningitis |
|
|
Spinal cord |
||
|
Amyotrophic lateral sclerosis |
||
|
Poliomyelitis |
||
|
Tetanus |
||
|
Peripheral nerve |
||
|
Phrenic nerve injury |
||
|
Botulism |
||
|
Guillain-Barre syndrome |
||
|
Muscle |
||
|
Electrolyte disorders (hypophosphatemia) |
||
|
Muscular dystrophies |
||
|
Diaphragmatic atrophy (severe emphysema) |
Acute hypercapnic respiratory failure
Definition
Acute hypercapnic respiratory failure is defined as a Paco2 greater than 45 to 50 mm Hg along with respiratory acidosis. Chronic failure is also marked by elevated Paco2 levels, but in patients with chronic respiratory failure, renal compensation tends to normalize the pH. The distinction of acute from chronic hypercapnic respiratory failure is important, because the two have clearly different prognostic significance and therapeutic implications.
Pathogenesis
Three processes, alone or in combination, can produce acute hypercapnic respiratory failure: a reduction in minute ventilation, an increase in wasted ventilation, and an increase in CO2 production.
Reduced minute ventilation can occur as a consequence of central nervous system disorders (e.g., brain injury or spinal cord lesions), peripheral nerve diseases (e.g., Guillain-Barre syndrome, botulism, myasthenia gravis or amyotrophic lateral sclerosis), muscle disorders (e.g., polymyositis or muscular dystrophy), chest wall abnormalities (e.g., thoracoplasty or scoliosis), drug overdoses, metabolic abnormalities (e.g., myxedema, hypokalemia), and upper airway obstruction. These disorders normally are associated with a normal A-aDo2 unless lung disease is also present.
Wasted ventilation is defined as the ratio of the dead space volume (vD) to the tidal volume (vT). An increase in wasted ventilation (i.e., an increased VD/VT ratio) is caused by overven-tilation of regions of the lung relative to their perfusion. This may occur in intrinsic lung diseases (e.g., emphysema, asthma, cystic fibrosis or pulmonary fibrosis) and in chest wall disorders associated with parenchymal abnormalities (e.g., scoliosis). Usually, these disorders are also associated with widened A-aDo2 gradients.
Increased CO2 production (Vco2) in hospitalized patients is usually a result of infection, trauma, burns, or other major stresses that lead to hypermetabolism. Agitation, myoclonus, or other causes of muscle activity can increase Vco2 and contribute to the development of hypercapnic respiratory failure. During refeeding, lipogenesis from the oxidation of carbohydrates can increase the metabolic respiratory quotient significantly. The respiratory quotient (i.e., the ratio of the volume of carbon dioxide released to the volume of oxygen consumed), which is normally 0.8, may rise as high as 2.0 in such cases; this basically doubles Vco2. In patients who have severe lung disease or are on fixed mechanical ventilation, acute hypercapnia may occur.
Diagnosis
Acute Hypoxemic Respiratory Failure
When the Pao2 rapidly falls below 40 to 50 mm Hg, harmful effects may be observed in various organ systems. Patients may experience headache, somnolence, confusion, and seizures. With more severe hypoxemia, permanent encephalopathy may occur. Cardiovascular sequelae from mild hypoxemia, including tachycardia and hypertension, may also develop. With severe hypoxemia, opposite effects may occur, such as bradycar-dia and hypotension. Patients who have disorders associated with hypoxia (i.e., decreased delivery of oxygen to the peripheral tissues) without concurrent hypoxemia can present with similar clinical signs and symptoms. Causes of hypoxia without hy-poxemia include anemia, decreased cardiac output, and carbon monoxide or cyanide poisoning.
Acute Hypercapnic Respiratory Failure
Signs and symptoms of hypercapnia depend not only on the absolute level of Paco2 but also on the rate at which the level increases. A Paco2 above 100 mm Hg may be well tolerated if the hypercapnia develops slowly and acidemia is minimized by renal compensatory changes. However, acute increases in Paco2 levels are associated with several neurologic sequelae, including increased cerebral blood flow and elevation in intracranial pressure.
Acute elevation in Paco2 to 80 to 90 mm Hg may produce many neurologic signs and symptoms, including confusion, headaches, seizures, and coma. A careful neurologic examination of a patient with acute hypercapnia may reveal agitation, coarse tremor, slurred speech, asterixis, and occasionally papilledema. These effects of hypercapnia on the central nervous system are fully reversible, as opposed to the potentially permanent neurologic sequelae that are associated with acute hypoxemia. An elevated Paco2 is also associated with myocardial depression, arrhythmias, hyperkalemia, and gastrointestinal bleeding.
Figure 1 The oxygen concentration of inspired gas is plotted against arterial oxygen tension (Pao2) for right-to-left shunts ranging from 0% to 50% of cardiac output. The arteriovenous oxygen content difference is assumed to be normal. Note that with right-to-left shunts in excess of 30%, there is virtually no increase in PaO2 with oxygen enrichment.
Table 2 Radiographic Appearance of Disorders That Cause Acute Respiratory Failure
|
Diffuse or Patchy Infiltrates Present |
Relatively Clear Lungs |
|
Acute respiratory distress syndrome |
Acute hypoxemic respiratory failure |
|
Pneumonia |
Pulmonary embolism |
|
Cardiogenic pulmonary edema |
Air embolism |
|
Atelectasis |
Fat embolism |
|
Aspiration |
Acute hypercapnic respiratory |
|
Interstitial lung disease |
failure |
|
Pulmonary contusion |
Asthma |
|
Alveolar hemorrhage syndrome |
Exacerbation of CoPD |
|
Metastatic or lymphatic spread of tumor |
Narcotic overdose |
|
Neuromuscular disease |
|
|
obesity-hypoventilation syndrome |
COPD—chronic obstructive pulmonary disease
Differential Diagnosis
The many disorders that cause acute respiratory failure can be classified into two categories on the basis of the presence or absence of infiltrates on chest radiographs [see Table 2].1 Most patients with acute respiratory failure have patchy or diffuse infiltrates and primarily develop hypoxemic respiratory failure. In patients whose chest radiographs show infiltrates, the most common causes of acute hypoxemic respiratory failure are pneumonia, cardiogenic pulmonary edema, noncardiogenic pulmonary edema (ARDS), atelectasis, aspiration, progressive interstitial lung disease, pulmonary contusion, and alveolar hemorrhage syndromes (e.g., Goodpasture syndrome, Wegener granulomatosis, and systemic lupus erythematosus).
Acute respiratory failure in a patient with a relatively clear chest radiograph can be either hypoxemic or hypercapnic. Acute hypoxemic respiratory failure in such patients is most often the result of pulmonary embolism or some other form of pulmonary vascular disease. Acute hypercapnic respiratory failure has five principal causes: (1) acute exacerbation of chronic obstructive pulmonary disease (COPD) or asthma, (2) neuromuscular dysfunction, (3) obesity-hypoventilation syndrome, (4) obstructive sleep apnea, and (5) drug overdose associated with a depressed respiratory drive.
Management
The three major steps in the management of acute respiratory failure are to (1) ensure an open airway, (2) restore oxygenation, and (3) maintain or restore ventilation to eliminate CO2.
Ensuring airway patency
Airway obstruction may develop in patients with depressed consciousness, dysfunction of the upper airway muscles caused by neuromuscular disease, or an inability to cough and clear secretions. Simple measures such as pushing the mandible for- ward, laying the patient in a lateral decubitus position, or placing a nasal trumpet or oral airway may alleviate upper airway obstruction caused by relaxed upper airway muscles. However, endotracheal intubation should be performed when bronchial secretions are excessive or the patient is at high risk for the aspiration of gastric contents. Emergency cricothyrotomy or tra-cheostomy is required only if there is glottic or infraglottic anatomic obstruction or if endotracheal intubation cannot be performed for other reasons, such as trauma to the oropharynx.
|
Table 3 Procedure for Direct Orotracheal Intubation |
|
1. Administer oxygen by face mask. Ensure arterial oxygen saturation > 95% before attempting intubation, if possible. |
|
2. Gather basic equipment: oxygen source, bag-valve device, suctioning device, endotracheal (ET) tube, blunt stylet, laryngoscope, 20 ml syringe. Have all equipment easily accessible. |
|
3. Place patient on nonmobile rigid surface. 4. If patient is in hospital bed, remove backboard and adjust bed height. |
|
5. Depress patient’s tongue with tongue depressor and administer topical anesthesia to patient’s pharynx. |
|
6. Position patient’s head in sniffing position by placing a small towel under the occiput and extending the head and neck. |
|
7. Administer intravenous sedation and neuromuscular blocker if necessary.* |
|
8. Have assistant apply Sellick maneuver (compressing cricothy-roid cartilage against vertebral bodies) to occlude esophagus and prevent regurgitation and aspiration of stomach contents. |
|
9. Grasp laryngoscope handle in left hand while opening patient’s mouth with gloved right hand. |
|
10. Insert laryngoscope blade on right side of patient’s mouth and advance to base of tongue, displacing tongue to the left. |
|
11. Lift laryngoscope away from patient at a 45° angle using arm and shoulder strength. Do not use patient’s teeth as a fulcrum. |
|
12. Suction oropharynx and hypopharynx if necessary. |
|
13. Grasp ET tube with inserted stylet in right hand and insert it into right corner of patient’s mouth, avoiding obscuration of epiglottis and vocal cords. |
|
14. Advance ET tube through vocal cords until cuff is no longer visible, then remove stylet. |
|
15. Inflate cuff with enough air to prevent significant air leakage. |
|
16. Verify correct ET tube positioning by auscultation of both lungs and the abdomen. |
|
17. Obtain a chest radiograph to verify correct position of the ET tube. |
‘Neuromuscular blockade can result in complete airway collapse and airway obstruction; personnel who are skilled in establishment of an emergency surgical airway should be available if paralysis is used.
An endotracheal tube may be passed orally or nasally. The oral route is easier and faster and allows the use of a larger-diameter tube. Proper patient positioning and preparation are required to optimize success with endotracheal intubation [see Table 3]. The larger tube facilitates suctioning of secretions and fiberoptic bronchoscopy and decreases the risk of sinusitis.2 Na-sotracheal intubation may be preferable in awake and unanes-thetized patients and in patients with limited mobility of the cervical vertebrae, such as that caused by ankylosing spondylitis or rheumatoid arthritis.
Complications of endotracheal intubation attempts occur immediately if the tube enters the esophagus. Other early complications of endotracheal intubation include cervical spine injury in patients with trauma or arthritis and injury to the teeth, nose, pharynx, larynx, and tracheobronchial tree. In addition, both prolonged tracheal intubation and tracheostomy can lead to the serious sequelae of tracheal stenosis or tracheomalacia, which can result from ischemic injury of the trachea by the inflatable cuff of the tube. Such difficulties have been reduced since the advent of high-volume and low-pressure cuffs and can be further reduced by not overinflating the cuff. Tracheostomies and endo-tracheal tubes are associated with the same long-term complications. Tracheostomy may provide greater comfort and more effective secretion removal and should be considered if mechanical ventilation is needed beyond 2 to 3 weeks.3