Polycystic ovary syndrome (PCOS) is defined as hyperandro-genism and reduced frequency of ovulation in the absence of other hyperandrogenic disorders. The clinical manifestations of PCOS in women with hyperandrogenism are cosmetic and reproductive. These patients present with hirsutism, acne, and irregular menstrual periods; ovulation may be infrequent or absent, and infertility can occur.
When assessing a patient with possible PCOS, the physician must rule out other conditions that can produce clinical hyper-androgenism, such as androgen-secreting tumors and nonclas-sic congenital adrenal hyperplasia. It is also necessary to identify patients whose hirsutism or acne results from increased sensitivity to normal androgen levels.
Epidemiology
PCOS is one of the most common endocrine disorders of women. In three population-based studies, the average prevalence of PCOS in women of reproductive age was reported to be about 6%.1-3 Among anovulatory women, the prevalence of PCOS is approximately 30%.4
Pathophysiology
In women, luteinizing hormone (LH) and adrenocorti-cotropic hormone (ACTH) normally drive the secretion of an-drogens by the ovaries and adrenal glands, respectively. PCOS is caused by abnormally increased secretion of LH, insulin, or both. Increased levels of those hormones stimulate the ovarian theca and stroma to produce excess quantities of androgen, including testosterone and androstenedione. Elevated ovarian androgen secretion tends to block the growth of a dominant ovarian follicle. Instead, many small follicles accumulate; these follicles range in size from 4 to 8 mm in diameter. In the absence of a dominant follicle, an LH surge is not triggered, and ovulation does not occur regularly.
About 95% of women with PCOS have elevated levels of LH secretion, and 50% have hyperinsulinemia.5 Those rates are influenced by body mass and genetics. In a population with a high prevalence of obesity, up to 100% of the women with PCOS will have hyperinsulinemia. Indeed, there may be two major phenotypes of PCOS: (1) lean women with markedly elevated levels of LH secretion and minimal or no insulin resistance and (2) obese women with slightly elevated or normal levels of LH secretion and insulin resistance and with markedly elevated levels of insulin secretion.
Women with PCOS show an abnormal increase in both the amplitude and the frequency of LH pulses in the early follicu-lar phase of the menstrual cycle.6 The elevated LH pulse frequency suggests an underlying increase in the pulse frequency of gonadotropin-releasing hormone (GnRH) secretion by the hypothalamus; this in turn suggests that PCOS results from a neuroendocrine disorder. The neuroendocrine mechanisms that raise GnRH pulse frequency are poorly characterized but may include alterations in hypothalamic opioid and cate-cholamine tone.
The insulin resistance in women with PCOS, as in other patients, has many possible causes, including genetic mutations in the insulin receptor and autoantibodies to the insulin receptor. The most common cause, however, is obesity. When pancreatic function is normal, resistance to the action of insulin in the liver, adipose tissue, muscle, and other insulin-sensitive tissues results in a compensatory and chronic hypersecretion of insulin. Laboratory studies suggest that insulin, especially in high concentrations, can stimulate ovarian androgen secretion.7 Why insulin resistance develops in muscle and fat but not the ovary remains unclear. It is possible that insulin stimulates ovarian androgen secretion indirectly by binding to the insulinlike growth factor-1 receptor in the theca and the stroma.
Hirsutism
At birth, all areas of the body except the scalp and eyebrows are covered with vellus hair, which is light colored; individual vellus hairs have a very narrow diameter. Androgens can transform vellus hair into terminal hair, which is dark; individual terminal hairs have a thick diameter. The amount of andro-gen necessary to stimulate this transformation depends on many factors, including the sensitivity of hair follicles to andro-gens and the site of the hair follicle on the body. When girls reach puberty, small quantities of adrenal and ovarian andro-gens stimulate the transformation of vellus hair in the pubic region and axilla into terminal hair. Substantially larger amounts of androgen are required to stimulate the growth of terminal hair in a male-pattern distribution—that is, on the face, chest, and abdomen.
The term hirsutism denotes an increase in terminal hair in a male-pattern distribution. Hirsutism varies considerably by race: it is rare in Asian women, for example, because their hair follicles are relatively insensitive to androgens. In some families, women may inherit heightened sensitivity of the follicles to androgens and therefore may experience a degree of hir-sutism at normal levels of circulating androgens.
Acne
The hair follicle is part of the pilosebaceous unit, which also contains a sweat or sebaceous gland. Androgenic stimulation of the pilosebaceous unit promotes not only hair growth but also the production of sebum. Blockage of the follicle by excessive sebum (in concert with inflammation from free fatty acids produced by bacteria and yeast in the follicle) produces acne. The pilosebaceous unit is both a target organ responsive to an-drogen stimulation and a site of androgen production and me-tabolism.8 Consequently, acne, like hirsutism, reflects both circulating androgen concentrations and genetic makeup.
Diagnosis
In PCOS, hyperandrogenism and oligo-ovulation or anovu-lation are present, but other hyperandrogenic disorders, such as androgen-secreting tumors and nonclassic adrenal hyper-plasia, are absent.
No single feature is pathognomonic for PCOS. The history, physical examination, and laboratory evaluation can all provide evidence that establishes the diagnosis of PCOS and that excludes other conditions associated with those features. Clinical evidence of hyperandrogenism includes hirsutism, acne, and menstrual irregularity. Laboratory evidence of hyperan-drogenism includes an elevated total or free serum testosterone concentration [see Table 1].
History
The history can provide key information in the differential diagnosis of androgen excess (and heightened androgen sensitivity) in women.
Age of Onset
In PCOS, oligomenorrhea, hirsutism, and acne typically begin in the perimenarchal or teenage years. The onset of severe hirsutism in menopause suggests an ovarian neoplasm.
Menstrual History
Patients with PCOS typically experience irregular menstrual cycles starting at menarche. Regular cycles are more consistent with familial or idiopathic hirsutism. A history of initially regular periods followed by onset of oligomenorrhea or amenor-rhea and hirsutism with virilization in adult life suggests an androgen-secreting tumor.
Pace of Progression of Hirsutism
In PCOS, hirsutism tends to progress slowly, over many years. Rapid progression to severe hirsutism suggests a viriliz-ing disorder from an androgen-secreting tumor. Patients with androgen-secreting tumors typically report other manifestations of virilization, such as deepening of the voice and secondary amenorrhea. Virilization will be evident on the physical examination (see below).
Family History
Approximately 50% of women with PCOS have a family history of PCOS, type 2 diabetes mellitus, or both. Alternatively, the history may disclose familial hirsutism, which begins at puberty and is accompanied by regular menstrual cycles and normal concentrations of circulating androgens. In the absence of a positive family history, hirsutism that is disproportionate to the patient’s racial background and is accompanied by regular periods is considered idiopathic, if laboratory test results are normal (see below).
Medication Use
Some medications appear to cause increased LH secretion and thus promote the development of PCOS. In particular, long-term use of the anticonvulsant valproate is strongly associated with the onset of PCOS.9
Cigarette Smoking
Women who smoke have higher concentrations of an-drostenedione and testosterone than do nonsmoking women.10 Hence, smoking may contribute to hyperandrogenism.
Physical examination
Hirsutism can be assessed objectively with the Ferriman-Gallwey scoring system [see Figure 1].11 Along with providing a baseline measurement of hirsutism, this system can also be used to follow the efficacy of treatment.
Various physical findings point to insulin resistance [see Table 2]. Excess weight is a major determinant of insulin resistance and hyperinsulinemia.12 Relative weight is best assessed by means of the body mass index (BMI), which is calculated by dividing the patient’s weight in kilograms by the square of the patient’s height in meters. Women with a BMI of greater than 27 (i.e., those who are overweight) are often insulin resistant and usually demonstrate hyperinsulinemia in response to a glucose stimulus. Women with a BMI of greater than 30 (i.e., those who are obese) are almost always insulin resistant. Women with a BMI of less than 22 are unlikely to be insulin resistant unless they have one of a relatively rare group of acquired or inherited lipodystrophic disorders.
Other physical findings that suggest insulin resistance are a waist-to-hip ratio greater than 0.85 and a waist circumference greater than 90 cm (35.5 in). The presence of acanthosis nigri-cans or achrochordons (skin tags) suggests hyperinsulinemia. The syndrome of hyperandrogenism, insulin resistance, and acanthosis nigricans (HAIR-AN syndrome) is the most severe form of the insulin-resistant phenotype of PCOS.
Unfortunately, the physical findings that are associated with insulin resistance tend to be specific but not sensitive. For example, patients with acanthosis nigricans are almost always insulin resistant, but many women with insulin resistance do not have acanthosis nigricans.13 Although the identification of severe insulin resistance on the basis of clinical manifestations may be relatively simple, the detection of mild insulin resistance may be difficult.
Table 1 Characteristics of Common Causes of Androgen Excess
|
Diagnosis |
Cause |
Ovulation Status |
Testosterone Level |
8 A.M. Follicular-Phase 17-Hydroxyprogesterone Level |
|
PCOS |
Elevated LH, serum insulin, or both |
Anovulation or oligo-ovulation |
Elevated (0.75-2 ng/ml) |
Normal (< 4 ng/ml) |
|
Idiopathic hirsutism |
Elevated production of an-drogen in the piloseba-ceous unit, or a mild form of PCOS |
Regular ovulation |
Normal (< 0.75 ng/ml) |
Normal (< 4 ng/ml) |
|
Adrenal hyperplasia |
Decrease in 21-hydroxylase activity because of a gene mutation |
Anovulation or oligo-ovulation |
Elevated (0.75-2 ng/ml) |
Elevated (> 4 ng/ml) |
|
Ovarian or adrenal tumor |
Disorder of cell growth |
Anovulation |
Markedly elevated (> 2 ng/ml) |
May be elevated |
LH— luteinizing hormone
PCOS—polycystic ovary syndrome
Figure 1 Ferriman-Gallwey scoring system for quantitating hirsutism. The 11 sites are graded from 0 (no terminal hair) to 4 (severe hirsutism). Women with a total score greater than 8 are considered hirsute.
A key aspect of the physical examination is a search for signs of virilization, such as clitoromegaly, increased upper body muscle mass, and male pattern baldness. These may indicate the presence of an androgen-secreting tumor.
Women with PCOS have enlarged ovaries, although the ovaries typically are not palpable on pelvic examination. If pelvic examination discloses a large, complex mass, the patient may have an adrenal or ovarian tumor.
Laboratory tests
The goals of the laboratory evaluation of hyperandrogenism are to rule out an adrenal and ovarian tumor, assess the severity of the androgen excess, and determine whether the source of the hyperandrogenism is adrenal or ovarian. Laboratory tests that are the most useful in the evaluation of hyperandrogenism include determination of the total serum testosterone level; determination of the 8 A.M. follicular phase 17-hydroxyprogesterone level; determination of the serum dehydroepiandrosterone sul-fate (DHEAS) level (if fertility is an issue); and determination of the serum prolactin level (if the patient has amenorrhea).
Testosterone
The serum testosterone concentration provides the best laboratory estimate of the severity of androgen overproduction. Either total or free testosterone can be measured. Total testosterone measurement is performed by all clinical laboratories; these tests are well standardized and are less expensive than free testosterone measurement. However, because the level of sex hormone-binding globulin decreases as testosterone production increases, the total testosterone level does not fully reflect the degree of hyperandrogenism, especially if the overproduction of testosterone is minimal. Many women with mild PCOS have a total testosterone level in the upper end of the normal range. If the total testosterone level is greater than 2 ng/ml (200 ng/dl), the patient probably has ovarian stromal hyperthecosis or an adrenal or ovarian tumor and needs a detailed evaluation, which should include imaging studies of the ovary and adrenal glands.
The free testosterone measurement is more sensitive in detecting mild androgen overproduction. Nevertheless, because the total testosterone adequately identifies women with marked androgen overproduction who need additional evaluation and because free testosterone is usually more expensive to assay, measurement of free testosterone is not routinely indicated.
|
Table 2 Physical Findings Associated with Insulin Resistance |
|
|
Body mass index* > 27 |
Acanthosis nigricans |
|
Waist-to-hip ratio > 0.85 |
Numerous achrochordons |
|
Waist > 90 cm |
(skin tags) |
*Calculated by dividing the patient’s weight in kilograms by the square of her height in meters.
17-Hydroxyprogesterone
Approximately 2% of women who present with hyperan-drogenism and oligo-ovulation or anovulation have nonclassic adrenal hyperplasia resulting from a 21-hydroxylase deficiency. The prevalence of this congenital disorder varies markedly among different ethnic groups, from below 1% in Hispanic populations to as high as 5% to 8% in Ashkenazi Jewish populations. The decision to screen for the disorder depends on the cost-benefit assessment of detection and the baseline prevalence of the disorder in the patient’s ethnic group.
If the 17-hydroxyprogesterone level at 8 A.M. (measured in the follicular phase of the menstrual cycle) is greater than 4 ng/ml, the patient probably has nonclassic adrenal hyperplasia resulting from a 21-hydroxylase deficiency. This diagnosis can be confirmed by a 60-minute ACTH stimulation test. The test utilizes a form of synthetic ACTH (cosyntropin) that contains the first 24 of the 39 amino acids of natural ACTH: 0.25 mg is given intravenously or intramuscularly, and the 17-hydrox-yprogesterone level is measured 60 minutes later. A post-ACTH 17-hydroxyprogesterone level greater than 10 ng/ml confirms the diagnosis of nonclassic adrenal hyperplasia resulting from a 21-hydroxylase deficiency.
DHEAS
DHEAS, an androgen prohormone that can be converted to testosterone in the periphery, is secreted almost exclusively by the adrenal glands. The normal DHEAS level in premenopausal women is 0.12 to 5.35 |g/dl. A DHEAS level above 10.70 |g/dl—that is, more than twice the upper limit of normal— should raise concern over a possible adrenal tumor. Many women with PCOS have a DHEAS level in the upper range of normal, for reasons that have not been clearly identified. In infertile women with PCOS whose DHEAS level is greater than 2 |g/ml, the combination of clomiphene and a glucocorticoid may result in higher pregnancy rates than clomiphene alone (see below).
Serum Prolactin
If the patient has amenorrhea, the laboratory workup should include an assessment of the serum prolactin level to rule out a prolactin-secreting pituitary tumor [see 16:I Amenorrhea]. Many clinicians also routinely measure serum follicle-stimulating hormone (FSH) and thyroid-stimulating hormone (TSH) levels in amenorrheic patients.
Serum Luteinizing Hormone
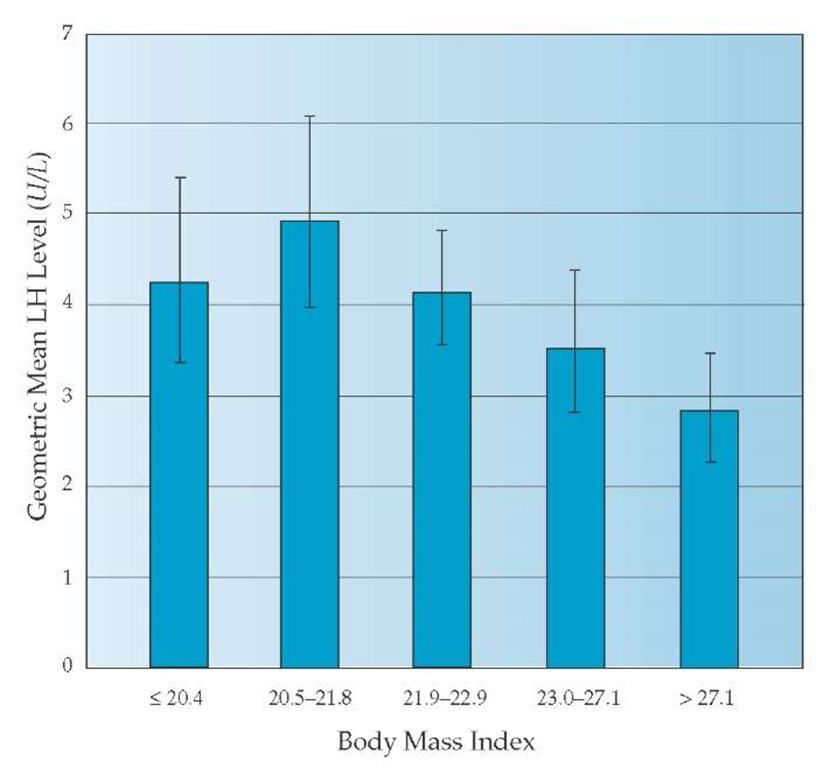
The measurement of serum LH presents a special problem in the laboratory evaluation of PCOS. In the research setting— using multiple serum LH measurements (every 10 minutes for at least 8 hours) and a precise and reliable LH assay—elevated LH levels can be documented in about 95% of women with PCOS. However, because LH secretion is pulsatile and the standard commercial assays are not as precise as research assays, measurement of LH in clinical practice is of only modest utility. An elevated LH level is reasonably specific for PCOS, provided the sample was not taken during a preovulatory LH surge. A normal LH value does not necessarily exclude PCOS, however, because the test sample may have been drawn when the patient was at the nadir of an LH pulse. Another important point is that as BMI increases, the normal range for LH decreases [see Figure 2].14 Nomograms that control serum LH for BMI are not widely available.
Pelvic Imaging
Demonstration of polycystic ovaries on pelvic ultrasonogra-phy is not essential for the diagnosis of PCOS. Pelvic imaging is indicated only if the ovaries are palpable on physical examination or the total testosterone concentration is greater than 200 mg/dl.
Tests for Detection of Insulin Resistance and Hyperinsulinemia
About 50% of women with PCOS have insulin resistance and hyperinsulinemia. There is no clear consensus on how to detect those conditions. A major problem is that the least resource-intensive laboratory techniques for diagnosing insulin resistance and hyperinsulinemia are specific but not sensitive. Elevation of the fasting serum insulin level or a fasting serum insulin-to-glucose ratio of less than 4.5 is almost always associated with insulin resistance, but many insulin-resistant women do not have fasting hyperinsulinemia. Laboratory techniques that are both specific and sensitive for detecting insulin resistance, such as euglycemic hyperinsulinemic clamp studies, are too complex and expensive for application in general clinical practice. Until both specific and sensitive laboratory tests that can be widely applied in practice become available, clinicians should use clinical findings and, if necessary, simple laboratory tests—such as assessment of fasting insulin levels or assessment of insulin response to an oral glucose challenge—to identify women with insulin resistance. Even nonobese women with PCOS have marked increases in circulating insulin after a glucose challenge [see Figure 3].15
Figure 2 Relationship between body mass index (BMI) and basal luteinizing hormone (LH) levels in women in the follicular phase of the menstrual cycle.