Refractory Idiopathic Thrombocytopenic Purpura
About 40% of ITP patients are characterized as refractory; they either remain severely thrombocytopenic after splenectomy and corticosteroid therapy or go into remission but later experience a relapse. Approximately 25% to 40% of patients will have a relapse 5 to 10 years after an initially successful splenectomy.16 Because serious hemorrhage is uncommon with platelet counts above 30,000/^l, it is often prudent to accept an incomplete response and not proceed to more toxic forms of management. Im-munosuppressive agents are generally the mainstay of therapy at this stage. However, it should be emphasized that there are no large randomized studies to address this difficult problem and that generally these patients should be referred to a hematologist.
There are several major treatment alternatives for refractory patients. Rituximab, a chimeric anti-CD20 monoclonal antibody, when administered at 375 mg/m2 I.V. once weekly for 4 weeks, produces a lasting and substantial response in approximately one third of patients with chronic refractory ITP; however, long-term follow-up is limited.22 The majority of the responses occur within 8 weeks after the first infusion. The therapy is generally well tolerated, with most of the side effects (i.e., fever, chills, mild hypotension, and bronchospasm) being infusion related and occurring during or after the first infusion. Rituximab produces a profound and prolonged peripheral B cell depletion in all patients, which can last for more than a year, but serious infection is rare. Azathioprine (100 to 150 mg/day orally) or, alternatively, cyclophosphamide (100 to 150 mg/day orally) plus prednisone (40 to 60 mg/day orally) can be given, but this therapy requires weekly monitoring of complete blood count and platelet count. Prednisone may be tapered and azathioprine or cyclophos-phamide adjusted to avoid severe leukopenia. A frequent mistake is to discontinue the therapeutic trial prematurely. Both aza-thioprine and cyclophosphamide are myelosuppressive and should be given in sufficient dosages to cause a mild leukopenia, with a white blood cell count of approximately 3,000/M, and both have been associated with development of myelodysplastic syndrome and acute myeloid leukemia. After 1 month, alternate-day prednisone therapy should be considered to avoid steroid side effects. Because of the concern of long-term marrow toxicity associated with azathioprine and cyclophosphamide, ri-tuximab should be considered the first-line therapy in refractory ITP patients, if treatment is indicated.
Another alternative is antibody therapy with intermittent courses of IVIg at the dosage schedules described (see above). The cost of this therapy and the usual short-lived response make it an unattractive choice. Anti-D antibody has been used successfully in Rh+(D+) patients with ITP; in the presumed mechanism of action, the antibody-coated red blood cells block Fc receptors on macrophages and prevent the accelerated removal of platelets. Other therapeutic options include vincristine, vinblas-tine,23 danazol,24 high-dose dexamethasone, cyclosporine, inter-feron alfa, and plasmapheresis.
In the refractory splenectomized patient, it is important to check for the continued presence of Howell-Jolly bodies and the possibility of an accessory spleen. The disappearance of Howell-Jolly bodies suggests the presence of a remaining accessory spleen or a regenerated spleen.
Patients with clinically significant thrombocytopenic bleeding can also benefit from fibrinolysis inhibitor E-aminocaproic acid (EACA). EACA can be given at 2 to 3 g orally four times daily until hemostasis is achieved.
HIV-Related Idiopathic Thrombocytopenic Purpura
HIV-1-related ITP appears to have a pathophysiology that is somewhat different from that of non-HIV-associated ITP, in that the antigenic specificity for the antiplatelet antibody is different. Two major antigenic determinants have been identified—a linear peptide in the platelet membrane GPIIIa and a cleavage product of talin, a platelet cytoskeletal protein, that can be generated by HIV-1 protease.25,26 In patients with HIV infection, platelets also contain increased amounts of IgG, IgM, complement, and immune complexes. Platelet survival is moderately short, and platelet production is impaired, especially at the later stages of the disease.27
The use of immunosuppressive agents in HIV-infected patients is hazardous. If the drop in the platelet count is modest, no therapy is needed. When the thrombocytopenia is severe, a short course of prednisone can be administered, followed by splenectomy.
Acute thrombocytopenic hemorrhage in HIV-associated ITP may be managed with high-dose IVIg, similar to the management of other ITPs. Chronic HIV-associated ITP may respond to oral zidovudine (AZT) or other antiviral therapies [see 7:XXXIII HIV and AIDS]. Anti-D antibody, dapsone, and interferon have also been used with some success.28-30 Patients who refuse splenectomy or who are thought to be poor surgical candidates may respond to low-dose splenic irradiation.31
Idiopathic Thrombocytopenic Purpura in Pregnancy
Mild thrombocytopenia, generally in the range of 110,000 to 150,000/ Ml and seldom below 70,000/ M, occurs in 5% of healthy pregnant women. When thrombocytopenia is observed for the first time during pregnancy, the differential diagnosis must include preeclampsia [see Table 3]. If other diagnoses can be excluded, the diagnosis is gestational thrombocytopenia (incidental thrombocytopenia of pregnancy); it requires no management.32 If the diagnosis of ITP is made, the the patients is considered to be at high risk for complications. The platelet counts should be monitored regularly and closely, especially in the third trimester, because in many pregnant women with ITP, thrombocytopenia progressively worsens over the course of the pregnancy. The therapeutic choices are limited because splenectomy may cause spontaneous abortion and immunosuppressive agents may damage the developing fetus; therefore, therapy is usually limited to corticosteroids or IVIg. Because corticosteroids increase the risk of preeclampsia and gestational diabetes, IVIg is the drug of choice. Generally, no treatment is required until the platelet count has fallen to 20,000 to 30,000/M or there is clinical bleeding. Typically, a single dose of IVIg (1 g/kg I.V. over 6 hours) will raise the platelet count to above 50,000/ M in the majority of patients, which will last for 3 to 4 weeks. Repeated doses can be given if necessary. In cases of severe thrombocytopenic hemorrhage, however, all of the available therapies should be used to protect the life and well-being of the mother.
Because the antiplatelet autoantibody in ITP has broad specificity and is almost always an IgG, it can cross the placenta and produce thrombocytopenia in the fetus. During a vaginal delivery, the pressure applied to the head of a thrombocytopenic fetus may induce an intracranial hemorrhage. Concern about this occurrence led many experts in the past to recommend early cesarean sections in women with a history of ITP or active disease. No data exist, however, to support this recommendation, and a much more conservative approach is now generally accepted. Most pregnant women with ITP undergo vaginal deliveries; cesarean sections are performed only for obstetric indications.
There is no correlation between maternal platelet count and the infant’s platelet count. A mother with a history of ITP who has a normal platelet count can deliver a thrombocytopenic neonate (~10% incidence). Alternatively, a thrombocytopenic mother can have an infant with a normal platelet count. Measurement of maternal antiplatelet antibody is of no clinical utility. The best predictor of thrombocytopenia in a neonate is the mother’s previous experience of giving birth to an infant with neonatal thrombocytopenia.33 Neonatal severe thrombocytope-nia—defined as a platelet count at birth that is less than 20,000/ m1—is uncommon (1% to 5% of births), and severe bleeding complications are rare (< 1%).34,35 The occurrence of neonatal severe thrombocytopenia is also unpredictable. The risk of in-tracranial hemorrhages in these infants is low (< 1%), and it cannot be reduced by cesarean section.36 Percutaneous umbilical blood sampling is generally not recommended. Many infants who are born to mothers with ITP will have a decrease in platelet count after delivery, with a nadir on day 2; the infant’s platelet count should be monitored daily for several days.32 Maternal ITP is not a contraindication to breast-feeding.
Thrombocytopenic Purpura with Lymphomas and Systemic
Lupus Erythematosus
Patients with SLE, Hodgkin disease, or non-Hodgkin lym-phoma can present with a clinical picture identical to that seen in ITP. The diagnostic approach and therapy are the same in these cases as they are in ITP. Splenomegaly with splenic sequestration, marrow infiltration with malignant cells, and recent anti-neoplastic or immunosuppressive therapy should be excluded. Patients with SLE or lymphoma may have Evans syndrome, in which ITP is associated with autoimmune hemolytic anemia. The management of Evans syndrome is the same as that of ITP and autoimmune hemolytic anemia.
Posttransfusion Purpura
Posttransfusion purpura (PTP) is characterized by acute onset of severe thrombocytopenia, often with a platelet count below 10,000/ ^l, accompanied by clinical bleeding. It may occur from 2 to 10 days after a transfusion of packed red blood cells or platelet-containing components. Almost all of the affected patients are multiparous women. Such disorders as septic throm-bocytopenia, DIC, and heparin-induced thrombocytopenia must be considered in the differential diagnosis. The thrombocytope-nia usually lasts for about 4 weeks. Because platelet transfusions are usually futile and sometimes precipitate severe systemic responses, they should be avoided if possible.
The pathophysiology of PTP is not completely understood. In most cases, the patient has been exposed to platelet alloantigens during pregnancy or as a result of a transfusion. Most patients with this disorder have antibodies to the human platelet anti-gen-1 (HPA-1), a polymorphic epitope present on platelet surface GPIIIa. The HPA-1 has two isoforms, HPA-1a and HPA-1b (previously PLA-1 and PLA-2). In the United States, approximately 98% of the white population, 99% of the African-American population, and 99% of the Asian-American population are homozygous for HPA-1. Patients in whom PTP develops are usually HPA-1a negative and HPA-1b positive. The patient has been sensitized to the HPA-1a antigen, most frequently during pregnancy, and reexposure to HPA-1a platelets during red cell transfusion leads to an anamnestic response and the destruction of the foreign platelets. It is puzzling that alloantibody directed against an antigen present on foreign platelets results in destruction of the patient’s autologous platelets, which do not express the HPA-1a antigen. There is evidence suggesting that the HPA-1a antigen becomes soluble and attaches to the HPA-1a-nega-tive platelets. Alternatively, exposure to foreign platelets may induce the formation of a true autoantibody against the endogenous platelets. The HPA-1a/HPA-1b polymorphism accounts for 80% to 90% of PTP. However, the presence of an alloantibody is necessary but insufficient for the development of PTP. Some patients with anti-HPA-1 a antibodies become refractory to platelet transfusions but do not have PTP.37 In addition, the incidence of PTP is far less common than might be predicted by the 1% to 2% of the general population who are homozygous for HPA-1b.
Confirmation of the diagnosis of PTP requires serologic studies demonstrating the presence of anti-HPA-1a antibody and a homozygous HPA-1b genotype. Several rapid platelet genotyp-ing techniques based on the polymerase chain reaction have been developed. Homozygous deficiency of platelet CD36 (glycoprotein IV) occurs in 3% to 5% of Asians and Africans, and al-loantibody against CD36 has also been found to be associated with PTP.38 There are no controlled clinical trials evaluating therapy for PTP because of the limited number of cases. IVIg, used at doses similar to those used in the treatment of ITP, is efficacious in about 80% of cases. Plasmapheresis is also efficacious, but it is more cumbersome than IVIg administration. Use of high doses of corticosteroids is not consistently effective.39 Transfusion of HPA-1a-negative platelets may provide some transient benefit in life-threatening bleeding situations.
Drug-Induced Immune Platelet Destruction
Drug-induced immune platelet destruction is indistinguishable from ITP. The bone marrow shows abundant megakaryo-cytes, and special laboratories can detect the presence of antidrug antibodies.
Quinidine and quinine purpura The pathogenic antibodies in cases of quinidine and quinine purpura develop as early as 12 days after exposure to the offending agent. In most cases, drug-dependent antibodies to platelet surface GPIb-IX have been identified in patients’ sera.41 The antibodies are drug dependent because they bind to the platelets only in the presence of quinine or quinidine. Presumably, the binding of the drugs to these platelet surface glycoproteins induces new antigenic sites on the proteins that are recognized by the antibodies.
The agent (quinidine or quinine) should be withdrawn in such cases. Neither corticosteroid therapy nor emergency splen-ectomy is of documented benefit in purpura induced by these agents. Plasmapheresis to remove the drug and antibodies would appear to be a logical treatment, but there are no systematic studies of its effectiveness. Transfused platelets are removed as rapidly as the recipient’s own platelets. Treatment with pred-nisone and IVIg in a dose similar to that used in ITP is recommended. Platelet transfusion after IVIg infusion may be given to control life-threatening bleeding.
A quinine-induced thrombocytopenia that is closely followed by the development of HUS has been recognized. Quinine-dependent antibodies to platelets, as well as to endothelial cells, have been found in patients’ sera.42 Even the small amount of quinine in tonic water seems to be sufficient to trigger recurrent bouts of the syndrome. Other drugs that may occasionally produce drug-dependent thrombocytopenia include dipyridamole and trimethoprim-sulfamethoxazole.43
Heparin-induced thrombocytopenia Heparin-induced throm-bocytopenia (HIT) is a frequent cause of drug-induced thrombo-cytopenia in hospitalized patients. Despite the presence of modest to moderate thrombocytopenia, HIT is rarely associated with bleeding but is associated with significant and sometimes fatal thrombosis [see 5:XIV Thrombotic Disorders].
Gold-induced thrombocytopenia Gold salt therapy for rheumatoid arthritis produces thrombocytopenia, which is sometimes severe, in 1% to 3% of patients. There are drug-induced autoantibodies that target platelet membrane GPV, but the presence of gold is not required for their reactivity.44 Most patients respond to therapy with 60 mg of prednisone daily. IVIg is also efficacious.
Cocaine-associated thrombocytopenia An ITP-like syndrome has been reported in intravenous cocaine users. They have been shown to respond to an approach similar to that employed in patients with ITP.
Thrombocytopenia caused by GPIIb-IIIa receptor antagonists Three parenteral GPIIb-IIIa antagonists—abciximab, epti-fibatide , and tirofiban—have been approved for use in the treatment of acute coronary artery syndrome and as adjunctive therapy in coronary angioplasty. In contrast to the low platelet counts in other types of drug-induced thrombocytopenia, patients who have low platelet counts resulting from GPIIb-IIIa receptor antagonists can develop acute, often profound thrombo-cytopenia within a few hours after drug administration. In patients receiving abciximab, thrombocytopenia occurs in about 1% after the first exposure. After a second exposure, the incidence of thrombocytopenia rises to 4%.46 The incidence of drug-induced thrombocytopenia associated with eptifibatide and tirofiban is probably also about 1% after first exposure.
The abrupt development of severe thrombocytopenia in patients who have never been exposed to these drugs initially suggested that platelets were being destroyed by a nonim-mune mechanism. However, accumulating evidence indicates that drug-dependent antibodies, which occur naturally, are the underlying cause. Preexisting anti-GPIIb-IIIa autoantibodies are present in these patients, and after the administration of the anti-GPIIb-IIIa antagonist, the binding of the drug to GPIIb-IIIa induces conformational changes in GPIIb-IIIa such that new epitopes are exposed that are recognized by the autoanti-bodies. These actions would explain the acute onset of profound thrombocytopenia.47
When thrombocytopenia develops (i.e., when platelet counts drop below 100,000/ M), the GPIIb-IIIa antagonist and any other potentially offending medications (e.g., heparin) should be discontinued immediately. Depending on the platelet count, it may not be advisable to discontinue antiplatelet agents such as aspirin or clopidogrel, because in such cases, patients are at high risk for acute coronary artery or stent thrombosis. If the platelet count drops below 10,000/ M, strong consideration should be given to platelet transfusion. In general, only one single-platelet transfusion is sufficient. There are anecdotal reports of acute coronary thrombosis associated with platelet transfusion in this setting when the platelet count climbs over 50,000/ m1 and the patient is off all antiplatelet agents. Thus, antiplatelet agents may need to be reinstituted. Because eptifibatide and tirofiban have very short half-lives and are cleared from the circulation within hours, the duration of thrombocytopenia is short, once the offending drugs have been discontinued. However, because abcix-imab has a much longer half-life—with inhibition of platelet function reported up to 1 week after drug discontinuance— thrombocytopenia can persist for 5 to 7 days. Platelet counts should be obtained in all patients before, as well as within 2 to 4 hours after, the initiation of an intravenous GPIIb-IIIa antagonist. It should be noted that a subgroup of patients develop delayed thrombocytopenia 5 to 8 days after abciximab administration. On the basis of limited published experience, it appears to be safe to administer eptifibatide or tirofiban to patients who are sensitive to abciximab, and vice versa.
Thrombocytopenia caused by metabolites of naproxen and acetaminophen Five patients have experienced thrombocy-topenia after taking naproxen and acetaminophen. In each case, antibodies that reacted with normal platelets in the presence of a known drug metabolite of naproxen or acetaminophen were identified.48 Therefore, the sensitizing agents are drug metabolites that formed in vivo.
ACCELERAtED ReMOVAL of PLaTELETS by NONIMMuNOLOGIC MeCHANISMS
There are several nonimmunologic causes for thrombocy-topenia. Blood vessel wall injury with increased thrombin generation and increased platelet activation and consumption occurs in several of these conditions.
Thrombotic Thrombocytopenic Purpura and Adult
Hemolytic-Uremic Syndrome
TTP and HUS encompass a group of clinical syndromes characterized by widespread platelet-fibrin thrombi deposition in the small arteries and arterioles and capillaries. Thrombotic mi-croangiopathy is a distinct feature of both TTP and HUS; however, the underlying pathogenetic processes in TTP and HUS may differ [see Pathogenesis, below]. Familial TTP/HUS is rare and usually occurs in the immediate postnatal period or infancy, although there are reported cases of delayed onset until the second to third decade of life. More frequently, TTP is either idiopathic or secondary to a variety of conditions [see Etiology, below].
Etiology TTP/HUS occurs spontaneously and is also associated with pregnancy, cancer, bone marrow transplantation, autoimmune diseases, and various drugs. In pregnancy, it resembles severe preeclampsia. In the postpartum period, the CNS manifestations may initially be confused with postpartum depression, with tragic results. Cases have been reported after a normal delivery and with abruptio placentae and preeclampsia.
Several drugs appear to cause TTP/HUS. These include chemo-therapeutic drugs (e.g., mitomycin C, bleomycin, and cisplatin), immunosuppressive agents (e.g., cyclosporine and FK506), the antiplatelet agent ticlopidine, oral contraceptives, and quinine. Anecdotal cases of TTP/HUS associated with clopidogrel, which is related to ticlopidine, have also been reported.
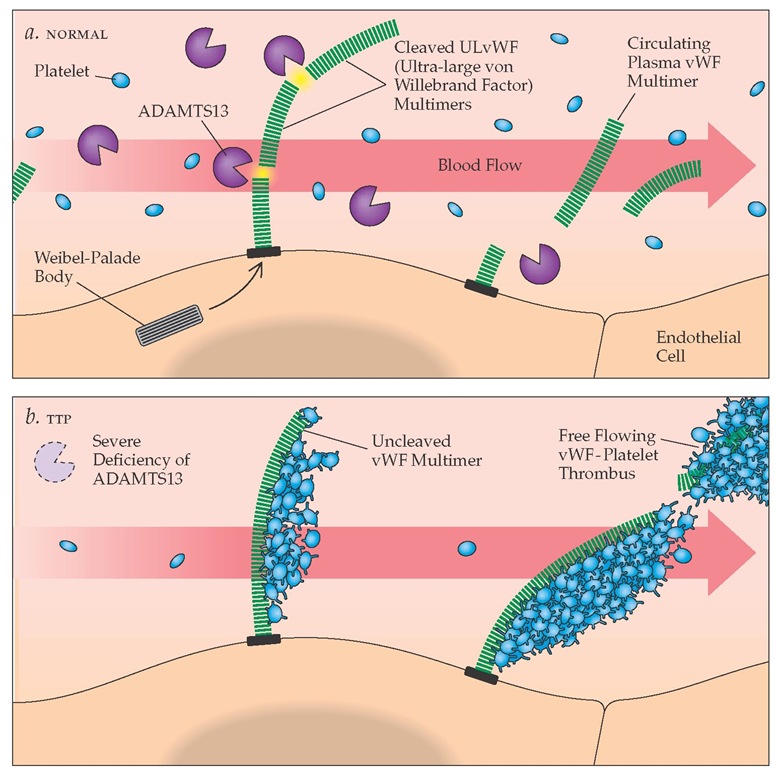
Pathogenesis There have been significant recent advances in the understanding of TTP, showing that the proper processing of von Willebrand factor (vWF) multimers plays a key role in its pathogenesis. vWF is an abundant plasma protein that mediates platelet adhesion to the subendothelium and serves as a carrier molecule for factor VIII [see 5:XII Hemostasis and Its Regulation]. vWF is synthesized by both megakaryocytes and en-dothelial cells. Monomers of vWF (280,000 daltons) are cross-linked by disulfide bonds to form vWF multimers, which are released into the circulation by endothelial cells and are stored within platelet a-granules and the Weibel-Palade bodies in endothelial cells. The stored vWF multimers can be released upon platelet or endothelial stimulation. These released vWF multimers are larger than plasma vWF multimers and are referred to as ultra-large vWF (ULvWF) multimers, with a molecular size up to 20 million daltons. Functionally, these are the most reactive vWF multimers. In 1982, ULvWF multimers were found in the plasma of patients with chronic relapsing TTP, giving rise to the hypothesis that TTP may result from the deficiency of a vWF-cleaving protease (depolymerase), which causes ULvWF multimers to circulate, contributing to the development of thrombosis.50 This hypothesis has been proved largely correct with the recent identification of the vWF-cleaving protease and the demonstration that deficiency of the vWF-cleaving protease activity is associated with TTP.
Figure 1 ADAMTS13 activity in normal and thrombotic thrombocytopenia purpura plasma. (a) In normal persons, ADAMTS13 enzyme molecules from the plasma attach to and then cleave the unusually large von Willebrand factor (ULvWF) multimers that are secreted in long strings from stimulated endothelial cells. (b) In patients with thrombotic thrombocytopenic purpura (TTP), a deficiency of ADAMTS13 prevents the cleavage of ULvWF multimers secreted by endothelial cells. Platelets carried by flowing blood adhere to the uncleaved ULvWF multimers, resulting in the development of platelet thrombi.
The vWF-cleaving protease has been identified as ADAMTS13 (a disintegrin-like and metalloprotease with thrombospondin type 1 motif 13).51 It is a novel metalloprotease that cleaves vWF monomer at a specific site (842Tyr-843Met) in the A2 domain. Current data indicate that ULvWF multimers are secreted from stimulated endothelial cells as a long "string" anchored on the endothelial cell surface. Plasma ADAMTS13 may attach, under flowing conditions within the blood, to the cell surface-bound ULvWF multimers (via the A3 domain in the vWF monomer) and cleave them into the vWF multimers that are normally found in plasma.52 Partial unfolding of the ULvWF multimers by shear stress forces in the blood presumably enhances the enzymatic cleavage process. Patients with familial TTP have hereditary deficiency of ADAMTS13,53 whereas patients with acquired idiopathic TTP have antibodies that inhibit the ADAMTS13 ac-tivity.54,55 In either case, the persistence of ULvWF multimers on the stimulated surface of the endothelial cell leads to the adhesion and subsequent aggregation of platelets, which in turn lead to the formation of platelet thrombi [see Figure 1]. (Presumably, platelets do not bind to the smaller plasma vWF multimers, because the platelet binding sites are not exposed in these vWF multimers). In addition to causing ischemic injury at the site of thrombi formation, it is likely that platelet thrombi resulting from aggregation of ULvWF multimers will break up and em-bolize downstream, resulting in further ischemic tissue damage.
The gene encoding ADAMTS13 is located on chromosome 9q34. More than 50 mutations in this gene have been identified in patients with familial TTP, most of which result in greatly reduced ADAMTS13 secretion in vitro.51 In many cases of acquired idiopathic TTP, an IgG autoantibody against ADAMTS13 is produced transiently, leading to severe deficiency of ADAMTS13 activity. ULvWF multimers are detectable in the plasma in some patients during the acute episodes but not after recovery.
Although the role of ADAMTS13 in the pathogenesis of TTP has been established, it appears that the majority of patients with HUS do not have severe ADAMTS13 deficiency, strongly suggesting that the pathogenesis of HUS is different.
ADAMTS13 as a screening assay The clinical utility of measuring ADAMTS13 is not established. In part, this is be cause there is no gold standard for its measurement; most of the current assays have long turnaround times and are not readily available. Furthermore, the sensitivity and specificity of ADAMTS13 deficiency for the diagnosis of TTP remains unclear. Decreased vWF-cleaving activity is found in many clinical conditions that are not associated with TTP, including cirrhosis, chronic renal insufficiency, ITP, DIC, SLE, leukemia, pregnancy, and the postoperative state; it is also associated with advancing age.56,57 In a prospective study involving 37 patients, severe deficiency in the ADAMTS13 level (< 5%) was found in 80% of patients with idiopathic TTP but in none of the patients with TTP associated with hematopoietic stem cell transplantation, cancer, drugs, or pregnancy.58 Thus, acquired TTP may be considered as either idiopathic or secondary; the former is generally associated with severe ADAMTS13 deficiency, whereas the latter is not. Among the patients with idiopathic TTP and severe ADAMTS13 deficiency, 44% had inhibitors. Other studies found an incidence of inhibitors in idiopathic TTP of 65% to 95%; however, part of the variation in study results may have to do with patient selection.
The reason why an inhibitor is not detectable in a substantial number of the idiopathic TTP patients is unclear. It is possible that the current assay is not sufficiently sensitive; alternatively, the assay may involve a nonneutralizing antibody that binds to ADAMTS13 and accelerates its clearance. New assays using a re-combinant vWF fragment as the substrate for the ADAMTS13 protease are in development and should help clarify some of these issues.
Clinical features and diagnosis The five major manifestations (pentad) of TTP are (1) severe microangiopathic hemolytic anemia associated with a very high serum lactic dehydrogenase (LDH) level and a blood smear showing the characteristic schis-tocytes and helmet cells; (2) moderate to severe thrombocytope-nia with increased marrow megakaryocytes, which indicates in-travascular platelet activation and consumption; (3) fever, which is occasionally quite high; (4) CNS signs and symptoms that can be quite mild initially with transient agitation, headache, and disorientation but that can sometimes progress explosively to hemiparesis, aphasia, seizures, focal deficits, coma, and death; and (5) renal disease, which is usually mild and produces moderate elevations of serum creatinine and urinary protein levels. It should be emphasized that many patients do not present with all these signs and symptoms. Patients with familial TTP/HUS typically exhibit a chronic relapsing course.
The adult form of HUS has features similar to those of TTP, although the pathophysiology may not be identical [see Pathogen-esis, above]. Common features of TTP and HUS include microan-giopathic hemolytic anemia, thrombocytopenia, and the presence of platelet fibrin thrombi in the small vessels. Renal involvement is uniformly severe in HUS, whereas CNS disease is less prominent than in TTP. There is a distinct form of HUS that occurs in children after gastrointestinal infection with Es-cherichia coli, usually serotype 0157:H7. These patients present with bloody diarrhea and hemorrhagic colitis. E. coli 0157:H7 or other strains elaborate verotoxins (also called Shiga toxins) that bind to specific receptors on the endothelial surface, causing cell damage and even cell death.63 Verotoxin-1 (VT-1) can induce the upregulation of various prothrombotic and proinflammatory adhesive molecules on endothelial cells.64 The microvascular en-dothelial cells are particularly susceptible because they have a high expression of VT-1 receptors, which may explain the propensity for thrombosis in the microcirculation. Antibiotic treatment of children with E. coli 0157:H7 infection increases rather than decreases the risk of HUS, presumably because it causes the release of verotoxins from injured bacteria in the intestine, making the toxins more available for absorption. Thus, routine treatment with antibiotics is not recommended.
Whereas a severe deficiency of ADAMTS13 (< 5%) may be specific for TTP,66 patients with severe ADAMTS13 deficiency may have prolonged asymptomatic periods. It is becoming clear that loss of ADAMTS13 activity, with an associated increase in circulating ULvWF multimers, is necessary but insufficient to cause an acute clinical TTP episode. The current data support the hypothesis that severe ADAMTS13 deficiency, be it from familial or acquired cause, predisposes the patient to thrombosis, and a second vascular inflammatory stimulus, such as infection, surgery, or pregnancy, causes the endothelium to increase its release of the stored UlvWF multimers, which, in the setting of grossly impaired processing, gives rise to ULvWF platelet thrombi in the microcirculation and clinical thrombosis.
Differential diagnosis Both TTP and HUS must be differentiated from SLE and from Evans syndrome. Microangiopathic hemolysis, neutrophilic leukocytosis, and a negative direct Coombs test (direct antiglobulin test) strongly suggest TTP or HUS. Coagulation tests usually reveal no significant abnormalities (i.e., no evidence of DIC); serum LDH is usually elevated. A marrow biopsy is generally not required but may show the characteristic, but not pathognomonic, platelet-fibrin hyaline thrombi in small arteries and arterioles.
Treatment Prompt institution of plasma exchange with fresh frozen plasma is the treatment of choice for TTP/HUS. In a large randomized trial by the Canadian Apheresis Group, intensive plasma exchange was more effective than plasma infusion in terms of patient survival (78% versus 63%).67 In that study, 1.5 times the calculated plasma volume was removed and replaced with fresh frozen plasma during each of the first 3 days of therapy; subsequently, one single-volume exchange a day was performed for a minimum of 7 days. Some investigators obtained good results with a daily single-volume exchange instead of a 1.5-volume exchange.68 It is reasonable to start with a daily single-volume exchange if the patient is clinically relatively stable, with moderate thrombocytopenia and no significant neurologic impairment. However, if the clinical situation worsens, more intensive double-volume plasma exchange (5,000 to 6,000 ml/day, or approximately 80 ml/kg/day) is indicated. Because vWF multimers are present in cryoprecipitate, cryosupernatant (i.e., fresh frozen plasma from which cryoprecipitate has been removed) can be substituted as replacement fluid when a patient is not responding to routine plasma exchange. One uncontrolled study showed increased benefit from this preparation as compared with fresh frozen plasma.69 Once therapeutic benefit has been achieved (as measured by restoration of normal CNS function, by rising platelet counts, and by falling LDH levels), the intensity and frequency of plasma exchange can be reduced to single-volume exchanges, first three times weekly and then twice weekly.
Although the importance of prompt plasma exchange has been established, the use of corticosteroids,70 aspirin, and dipy-ridamole has not been tested in prospective clinical trials. With the observation of autoantibody against ADAMTS13 as a significant cause of acquired idiopathic TTP, rituximab (375 mg/m2 I.V. once weekly for 4 weeks) has been tried and reported to be effective, although the overall experience is still limited.71-73 Because pheresis tends to lower the platelet count in a patient who is already thrombocytopenic, the problem of platelet transfusion arises. Some investigators have observed that platelet infusion may lead to exacerbation of TTP,74 whereas others use platelet transfusions as required.
In the previously described prospective study of patients with severe deficiency in ADAMTS13 activity (see above), plasma exchange proved to be a useful therapy; among the patients with idiopathic TTP and severe ADAMTS13 deficiency without detectable inhibitors, the majority responded to plasma exchange with complete remission and a rise in the ADAMTS13 level.58 However, in patients with mild ADAMTS13 deficiency and high inhibitor levels, plasma exchange was not effective in reducing the inhibitor titer or in increasing the ADAMTS13 activity. Nevertheless, some of these patients had a favorable clinical response, including resolution of thrombocytopenia and cessation of hemodialysis. Among the patients whose TTP was not idio-pathic, response to plasma exchange was variable. Of note, mortality in patients with idiopathic TTP with severe ADAMTS13 deficiency has been shown to be 15% to 20%, whereas mortality in patients with nonidiopathic TTP has been much higher, at 55% to 60%.58,60 Many of the patients in the latter group have had serious underlying disease and comorbidities, such as hema-topoietic stem cell transplantation, which likely has contributed to the high mortality.
Management of acute TTP should therefore depend on the clinical manifestations and course of the disease.75 In all cases, plasma exchange is first-line therapy. Patients who respond promptly and completely to plasma exchange—which most likely will be those patients with idiopathic TTP and very low or nondetectable levels of inhibitors—may not need any further treatment. For patients who show a suboptimal response—such as an initial rise in platelet counts or a recurrence in thrombocy-topenia when the plasma exchange treatments are decreased— glucocorticoid is indicated. For patients who experience a more aggressive course (e.g., those with severe neurologic abnormalities or those who do not respond to the initial plasma exchange with or without steroid therapy), more intensive immunosup-pressive therapies, such as rituximab, should be considered.
Microangiopathy may persist for weeks or months after all other evidence of disease has subsided. In a large follow-up study of TTP patients, about one third of patients who entered remission had a relapse over a 10-year period.76 The risk of recurrence is largely restricted to patients with severe ADAMTS13 deficiency—primarily, patients with idiopathic TTP. Relapse seldom occurs in patients who have TTP in association with hematopoietic stem cell transplantation or drugs. Conflicting data have been reported regarding patients who have had relapses after TTP associated with pregnancy.
Most experts treat adult HUS in a manner similar to that for TTP. However, the response to plasma exchange appears to be less favorable in HUS than in TTP, which may be consistent with the recent finding that the ADAMTS13 level is generally not diminished in HUS.