Stages I and II Disease
Surgery Before a patient with stage I or II lung cancer undergoes surgery, the physician must undertake a determination of operability, which includes assessment of the medical risk of thoracotomy, as well as the risk of removal of the requisite pulmonary parenchyma. Cardiopulmonary disease, which is usually a consequence of tobacco use, is the major cause of postoperative morbidity and mortality in patients with stage I or II disease and, consequently, is the most significant medical factor in determining operability.
Pulmonary function testing and arterial blood gas analysis are used to determine the feasibility of pulmonary resection. Postoperative pulmonary function is estimated on the basis of the patient’s preoperative function and the projected resection of pulmonary parenchyma. Resection is generally contraindi-cated when the predicted postoperative forced expiratory volume at 1 second (FEVX) and forced vital capacity are less than 30% of predicted values. In patients with marginal results on preoperative pulmonary function studies, ventilation-perfu-sion scanning may be required to determine resectability. Postoperative FEV1 may be predicted after assessing the contribution to overall pulmonary function made by each lung and by specific pulmonary segments.
In patients who have a history of angina or whose preopera-tive electrocardiogram shows ischemia or arrhythmia, ra-dionuclide evaluation of myocardial perfusion or function is indicated. Normal results with these studies reliably exclude significant coronary artery disease; patients with positive results should undergo coronary arteriography. Recent myocar-dial infarction, uncontrolled heart failure, or uncontrollable arrhythmia precludes thoracotomy for pulmonary resection.
The final determination of resectability is made at thoracoto-my. Contraindications to pulmonary resection at the time of thoracotomy include pleural metastases, extensive mediastinal lymph node involvement (N3 disease), or direct extension of the tumor to critical structures (T4 disease). In addition, pulmonary resection is aborted if the extent of resection required would leave the patient with inadequate pulmonary reserve, as determined by preoperative pulmonary function studies.
Four main oncologic principles guide resection for lung cancer: (1) removal of the entire tumor with an anatomically complete portion of lung (lobectomy or pneumonectomy), to ensure removal of all intraparenchymal lymphatic drainage; (2) en bloc resection of adjacent structures, if technically possible, including the chest wall, diaphragm, and pericardium, without transgressing the tumor; (3) assessment of questionable resection margins by frozen-section analysis to optimize the potential for complete resection; and (4) sampling or complete dissection of all accessible mediastinal lymph nodes to improve staging.
In patients with small (< 3 cm) peripheral nodules and no mediastinal lymphadenopathy by CT criteria (i.e., no lymph nodes > 1 cm in diameter), the procedure of choice is lobecto-my and mediastinal lymph node dissection. Less extensive resection, such as wedge resection or segmentectomy, has been shown to be associated with significantly greater risk of local recurrence and cancer-specific death.27 In patients with T2 or T3 tumors or with mediastinal adenopathy on chest CT, cervical mediastinoscopy should be performed before exploration for pulmonary resection.
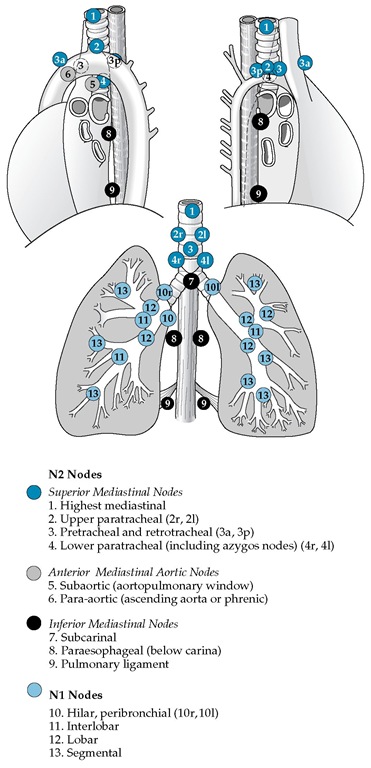
Figure 2 Draining lymph node sites (nodal stations) in the chest that can be involved by lung cancer are noted. Clinical staging of cancer of the mediastinum is carried out by CT scanning; surgical staging is performed by mediastinoscopy, mediastinotomy, thoracoscopy, or, sometimes, thoracotomy. A lymph node larger than 1 cm on CT scan is considered abnormal, but cancer involvement must be proved by biopsy. The upper paratracheal nodal stations are designated as 2r (right) and 2l (left); the lower paratracheal nodal stations are designated as 4r and 4l. Stations 8r, 8l, 9r, and 9l are contiguous with the mediastinum, but positive nodes in these sites are not common. Station 10 nodes, when confirmed as positive by mediastinoscopy, are classified as tracheobronchial angle nodes (10r and 10l) and signify mediastinal involvement. In the drawing, r is right; l, left; a, anterior; and p, posterior.
Table 6 Therapy and Prognosis for Non-Small Cell Lung Cancer
|
Stage (%) |
Surgery |
Radiotherapy |
Chemotherapy |
Five-Year Survival (%)* |
|
I (10) |
T1N0 (coin lesion): lobectomy (poor pulmonary function, segmental resection) |
None |
Research |
T1N0: 45-80 |
|
T2N0: lobectomy |
||||
|
II (10) |
T1N1 or T2N1: lobectomy; pneumonectomy |
May reduce local recurrence but |
T1N1: 20-52 |
|
|
usually required when hilar nodes are grossly involved |
does not affect survival |
Research |
T2N1: 20-40 |
|
|
IIIA (20) |
T3 (potentially resectable): radical resection |
Used after surgery; may reduce |
T3 (chest wall): 30-55 |
|
|
of chest wall lesions; used after RT of Pancoast tumors |
local recurrence; used preoper-atively for Pancoast tumors |
and surgery* |
T3 (Pancoast tumors): 20-40 |
|
|
N2 (potentially resectable): radical resection of early intranodal disease; not indicated for extranodal or fixed, matted nodes |
Used after surgery; may reduce local recurrence; used preoper-atively in some patients with early intranodal disease |
Combined with RT and surgery* |
N2: 10-50 |
|
|
IIIB (20) |
T4, N3, or both (unresectable) |
Standard treatment for palliation of pain, hemoptysis, atelectasis, hoarseness, SVC syndrome |
Combined with RT* |
Median, 30 wk |
|
IV (40) |
Used rarely for isolated metastases |
Useful for palliation of pain or other local problems |
Response rates of 30%-40%; prolongation of survival |
Median, 13-18 wk |
^Survival is higher in patients staged by surgery than in patients staged clinically.
*Randomized trials show prolonged survival when chemotherapy is added to radiotherapy, surgery, or both. RT—radiotherapy SVC—superior vena cava
Chemotherapy The benefit of chemotherapy for patients with stage I or II NSCLC is controversial. In clinical practice, treatment recommendations for such cases do not routinely include chemotherapy. Nevertheless, in reviewing treatment options with these patients, it is important to discuss the evolving data from clinical trials of chemotherapy.
A meta-analysis found that adjuvant treatment with alkylating agents in this setting resulted in a 5% decrease in survival, compared with surgery alone (P = 0.005).28 Cisplatin-based regimens were associated with a 5% improvement in 5-year survival, but this effect did not reach statistical significance (P = 0.08). On the other hand, the clinical trials included in this meta-analysis were performed between 1965 and 1991, and both chemotherapy and supportive care have improved significantly since that time. Therefore, randomized trials of adjuvant chemotherapy versus supportive care alone in stage I and II NSCLC are currently under way. An intergroup trial sponsored by the Cancer and Leukemia Group B (CALGB) is comparing carboplatin and paclitaxel with supportive care in patients with stage IB disease. An intergroup trial sponsored by the National Cancer Institute of Canada (NCI-C) has compared cisplatin and vinorelbine with supportive care alone in patients with stage IB, IIA, and IIB disease; results of this trial are pending. The International Adjuvant Lung Cancer Trial (IALT), in which patients with stages I through IIIA resected NSCLC were randomized to cisplatin-based chemotherapy versus observation, found a 4% absolute improvement in survival with chemotherapy.29 It is hoped that ongoing studies will confirm this benefit. In the meantime, the option of adjuvant chemotherapy should be discussed with patients after definitive surgical resection.
Another treatment strategy for stage I and II disease is the use of induction (preoperative) chemotherapy. Because of encouraging results from a phase II trial in patients with completely resected stage IB, IIA, or IIB NSCLC,30 the Southwest Oncology Group is leading a prospective, randomized trial of induction chemotherapy with three cycles of paclitaxel and carboplatin followed by surgery, compared with surgery alone. Other randomized clinical trials of both induction and adjuvant chemotherapy are currently being conducted for patients with stage IB, IIA, or IIB NSCLC. Eligible patients should be encouraged to enroll in these clinical trials, so that oncologists can determine whether chemotherapy is beneficial in this setting.
Radiation therapy Surgery is the treatment of choice for stage I NSCLC, but patients with medical contraindications to surgery can be treated with radiation therapy alone. Retrospective studies of such cases have shown 5-year survival rates ranging from 10% to 30%.31 Better local control was found in patients with smaller tumors (< 3 cm) and in those treated with higher doses of radiation (> 65 Gy). Consequently, recommended radiation doses range from 65 to 70 Gy; the total dose is typically given in 2-Gy fractions. Omission of regional nodal areas from the treatment fields has been found to reduce morbidity and has resulted in a nodal failure rate of only 4% to 9%. Therefore, in most cases, the primary tumor is treated with a standard margin of 1.5 to 2 cm. It is important to take into account any movement of the tumor from respiration, and this is best done under fluoroscopy. Unfortunately, most patients treated with radiation therapy succumb to recurrent lung cancer, and at least 60% experience local failure.
Stage Ill-Operable Patients
Patients with stage IIIA disease who appear to be candidates for surgical resection but in whom mediastinoscopy shows ip-silateral mediastinal lymph node involvement are evaluated for induction therapy (chemotherapy alone or chemotherapy and radiation therapy). Induction therapy with systemic chemotherapy has the potential to treat occult metastatic disease, which is common in patients with stage IIIA disease, even when organ-specific scans are negative. Three randomized, prospective trials that compared induction chemotherapy before surgery with surgery alone in patients with operable stage IIIA NSCLC were small in sample size, but all demonstrated benefit from induction chemotherapy, with at least a doubling in 3-year survival.
The addition of radiation therapy to induction therapy may improve local control in conjunction with surgical resection and may also decrease distant metastatic spread during therapy.27 A prospective study has suggested that chemoradiation before surgery is beneficial in patients with stage IIIA NSCLC.33 A randomized intergroup comparison of chemoradiation alone with chemoradiation followed by surgery has been conducted35; preliminary results suggest longer disease-free survival in the surgical arm but higher initial mortality, which complicates the analysis. Important questions remain about induction therapy, including the following: What are the optimum agents for chemotherapy? Should chemotherapy be used alone or in combination with radiation treatment? Does radiation therapy or surgery provide better local control? Should all three modalities of therapy be utilized? To answer these questions, enrollment of patients with stage IIIA NSCLC in clinical trials is critical.
After induction therapy, staging studies are repeated. Repeat mediastinoscopy is useful for reassessing the mediastinal lymph nodes, although this is more difficult than the primary procedure. Alternatively, the ipsilateral mediastinal lymph nodes may be assessed at exploratory thoracotomy. Pulmonary resection is not recommended if the involved lymph nodes have not responded to induction therapy or if there is evidence of disease progression, because the prognosis for extended survival is dismal in such patients.
Patients with involvement of the chest wall, diaphragm, or pericardium may be surgical candidates but only if the tumor can be completely resected. Incomplete resection of NSCLC provides no curative or palliative benefit.
Postoperative radiation therapy The treatment of patients found to be in stage II or III after resection is somewhat controversial. These patients are at a high risk for local and regional recurrences after surgery alone; however, they also have a very high likelihood of distant disease.36 In a study of patients with stage II or III disease who had undergone a complete resection and were randomized to receive radiation therapy or no further treatment, the patients who received radiation therapy were found to have a significantly lower rate of local failure (3% versus 21% for patients who did not receive postoperative radiation).37 However, there was no evidence of a survival benefit for the patients receiving postoperative radiation. Two caveats regarding this study are that it included only patients with squamous cell carcinoma and that most of the patients in the study had N1 nodal disease, precluding a valid subgroup analysis of the relationship between nodal status and survival.
A meta-analysis of nine published and unpublished randomized trials of postoperative radiation therapy—which included 2,128 patients with stage I, II or III lung cancer treated from 1966 to 1994, largely with cobalt radiation techniques—found that overall, mortality was approximately 7% higher for patients who received postoperative radiation therapy.38 On subgroup analysis, the adverse effect was most apparent in patients with N0 and N1 disease; survival of patients with N2 disease was the same in the two groups. The results of this study indicate that radiation therapy is detrimental to patients with early stage (I and II) lung cancer that has been completely resected; the question of whether postoperative radiation therapy benefits patients with N2 disease remains unanswered. This meta-analysis has been critiqued for its inclusion of patients treated with a wide variety of radiation doses and techniques, many of them now outdated, which may have skewed the data from showing a survival benefit with postoperative radiation therapy.
In summary, the use of postoperative radiation therapy in patients with stage II or III NSCLC yields a significant increase in local control, which may be particularly important in patients with positive surgical margins. However, because of the high frequency of metastatic disease in these patients, postoperative radiation therapy appears to provide no survival benefit. Patients offered postoperative radiation therapy should clearly understand that its goal is improved local control. Meanwhile, the possible role of combination radiation therapy and chemotherapy as an adjuvant to surgery is the subject of ongoing clinical trials.
Adjuvant chemotherapy As in stage I and II NSCLC, the role of adjuvant chemotherapy in resected stage III disease is not well supported by the results of randomized clinical trials, although the IALT results (see above) may provide such sup-port.28,29 On the other hand, a trial comparing adjuvant cisplatin and etoposide plus radiation with radiation alone in resected stage II and IIIA disease found no survival advantage for the group receiving adjuvant chemotherapy.39
Stage IIIB disease A small subgroup of patients with stage IIIB NSCLC may be candidates for surgical resection. In general, T4 tumors are considered unresectable; however, there are two exceptions to this generalization. First, patients with a single satellite nodule within the same pulmonary lobe as the primary tumor are offered resection if the disease is apparently resectable by lobectomy and the results of both medi-astinoscopy and organ-specific staging studies are negative. Second, in rare cases of very limited involvement of the vena cava, main pulmonary artery, or aorta by the primary tumor, en bloc resection and vascular reconstruction may be offered to selected patients; long-term survival in such cases ranges from 10% to 20%.
Some patients with contralateral mediastinal lymph node involvement (N3) are treated with induction therapy followed by surgical resection. However, the standard of care for these cases is chemotherapy and radiation therapy.
Stage Ill-Inoperable Patients
Radiation therapy Without treatment, most patients with stage IIIB NSCLC will succumb to their disease within 1 year. Radiation therapy does result in an improved outcome, with up to 20% of patients surviving 2 years and up to 5% surviving 5 years, but there is a high likelihood of local recurrence, ranging from 25% to 50% in some studies.40 Distant recurrence is also common. An analysis of several studies reveals that patients with weight loss greater than 5%, performance status of less than 80%, and higher T and N stage have the worst prognosis.41
In an attempt to increase the efficacy of radiation therapy, fractionation schemes have been tested, including treatments given two or three times daily. A randomized trial, performed in Europe, is comparing the effectiveness of continuous hyper-fractionated accelerated radiation therapy (HART), given three times a day, with conventional radiation treatment. Results of this protocol reveals a statistically significant survival benefit of 9% at 3 years for patients treated in the continuous HART arm.42 There is also a significant increase in local control, with 7% fewer failures at 3 years in the CHART arm. These benefits are most prominent in patients with squamous cell carcinoma. The results of several trials indicate that the addition of chemotherapy to radiation therapy leads to an improved survival. A multigroup, randomized study found that patients with unresectable cancer who received chemotherapy and radiation therapy in combination had a statistically improved overall survival compared with those who received only radiation either once or twice daily. For chemoradiation, standard radiation, and hyperfractionation, 3-year survival rates were 17%, 11%, and 9%, respectively, and median survival rates were 13.2 months, 11.4 months, and 12 months.43 In a comparison of HART with once-daily radiation therapy after induction chemotherapy,the 2-year survival was 40% for the HART arm, compared with 33% for the standard radiation therapy group; toxicities, particularly esophagitis, were also increased.44
Clinical trials are currently evaluating the use of three-dimensional treatment planning systems to increase the dose of radiation therapy delivered to the primary tumor. Preliminary results show that dose escalation is feasible and does not lead to increased toxicity and that outcomes are comparable to or better than historical controls.45
Chemoradiotherapy The standard treatment for inoperable stage III NSCLC is a combination of chemotherapy and radiation therapy. The chemotherapy should be a platinum-based combination regimen; the radiation should be given at conventional doses, generally 66 Gy. The use of chemoradio-therapy in these cases is supported by level I-A evidence.46 For unresectable stage III disease, chemotherapy plus radiation therapy is appropriate for patients with a good performance status (an Eastern Cooperative Oncology Group [ECOG] score of 0 to 1 or, possibly, 2).
The optimal strategy for coordinating chemotherapy with radiation therapy is evolving. Possibilities include chemotherapy before, during, or after radiation treatment. Promising results have been reported from a recent randomized phase II trial of induction chemotherapy followed by concurrent chemotherapy and radiation treatments.47 Median survival in this trial was approximately 18 months, which compares favorably with the 13 to 14 months reported in previous trials. This trial utilized cisplatin-based chemotherapy along with vinorelbine, pa-clitaxel, or gemcitabine, which are all agents with documented benefit in stage IV NSCLC (see below). A randomized phase III trial is needed to determine whether the apparent improvement in median survival stems from the sequencing of chemotherapy and radiation treatment, the use of the new agents, or both.
Concurrent administration of chemotherapy and radiation therapy has also been found to be beneficial. In a randomized trial of chemotherapy with mitomycin, vinblastine, and cis-platin given either before or along with thoracic radiation, the 2-year survival rates with sequential and concurrent treatment were 27% and 35%, respectively.48 Subsequent trials point to a survival benefit from concurrent chemotherapy and radiation treatment compared with sequential therapy, as well as an increase in toxicity, particularly esophagitis and pneumonitis.
Treatment Strategies in Stage III Disease
The optimal approach to management of stage III NSCLC remains undefined. At present, it is clear that chemotherapy, when used in combination with surgery or radiation treatment, can improve patient survival in both operable and inoperable disease. In patients with inoperable stage III NSCLC, long-term survival is better with platinum-based combination chemotherapy and radiation therapy than with either modality alone. Every attempt should be made to enroll patients in clinical trials to further clarify the optimal strategy.
Because the benefits of combination therapy have been largely demonstrated in only younger patients with higher performance status, physicians should use caution in applying these approaches to elderly patients or those with poor performance status. In the absence of data from elderly and poor-performance patient populations, low-dose chemotherapy and concurrent radiation can be considered. An alternative strategy that may result in less toxicity from esophagitis would be the use of combination chemotherapy followed by radiation treatment. This strategy allows individualization of treatment based on the patient’s tolerance of induction chemotherapy. However, older patients with good performance status should not be denied the potential benefit of combined-modality therapy. In this setting, it would appear that the most appropriate choice for chemotherapy is a cisplatin-based or carboplatin-based regimen with one of the newer agents used in the management of stage IV disease.