Diagnosis
Clinical Manifestations
Onset of scrub typhus typically occurs 7 to 10 days after the person is bitten by an infected mite. The illness may begin gradually or abruptly. In either case, headache, anorexia, malaise, chills, and fever are prominent early symptoms. Approximately one half of patients with scrub typhus develop a characteristic macu-lar or maculopapular rash. In severe cases, this rash may become petechial. Rash from scrub typhus typically spares the face.
A localized necrotic skin lesion or eschar is a hallmark of scrub typhus [see Figure 3]. Eschars typically occur at the site of the infected chigger bite and may appear before the onset of systemic symptoms. Some patients have multiple eschars. In various case series, eschars have occurred in as few as 40% and as many as 88% of patients with scrub typhus.26
Generalized lymphadenopathy occurs in most patients with scrub typhus; some patients also have splenomegaly. Respiratory involvement occurs more often in scrub typhus than in other rickettsial diseases, and cough may be present in as many as one half of patients. As in other rickettsial diseases producing vas-culitis, scrub typhus may be marked by neurologic involvement, which may manifest as aseptic meningitis, seizures, or focal neurologic abnormalities. In some patients, neurologic abnormalities are the dominant symptoms.
Diagnosis
The initial diagnosis of scrub typhus must be based on clinical and epidemiologic features, because serologic testing is not reliably positive in the early phases of illness. Convalescent antibodies, which are best detected by an IFA technique, occur in the majority of patients between 10 and 20 days after onset of illness. Because more than one strain of O. tsutsugamushi is capable of causing scrub typhus, a battery of antigens should be used to detect convalescent antibodies. In addition to IFA testing, an enzyme-linked immunosorbent assay (ELISA) and a dot-blot im-munoassay have been developed to diagnose scrub typhus.
Although not commonly done, biopsy of the generalized rash or the eschar can help establish the diagnosis of scrub typhus.
Figure 3 Eschar at the site of a mite bite in a patient with scrub typhus.
Examination of the biopsy sample will reveal vasculitis with a perivascular collection of lymphocytes and macrophages.
Culture of O. tsutsugamushi can be performed in specialized centers with the necessary laboratory facilities and diagnostic reagents; a PCR-based test has also been developed. However, such tests are rarely available in regions of the world where scrub typhus is endemic.
Differential diagnosis
Scrub typhus is one of the classic causes of tropical fever in the Pacific Rim, where it is well known to be easily confused with malaria, dengue, or typhoid fever. Coinfection with scrub typhus and leptospirosis has been described in agricultural workers in Thailand. One of these workers, who was treated with penicillin only, died of respiratory failure attributed to untreated scrub typhus infection.27
Treatment
The treatment of scrub typhus is the same as that of most other rickettsial diseases [see Table 2]. Doxycycline is typically the agent of choice. Regimens of doxycycline as short as 1 day have been advocated for the therapy of scrub typhus, but a small number of patients treated with this short course suffer re-lapse.28,29 To prevent relapse, most experts recommend a 3- to 7-day course of therapy with doxycycline.
In the mid-1990s, strains of O. tsutsugamushi with reduced susceptibility to tetracycline were reported in Thailand.30 Azi-thromycin may be effective against strains with reduced susceptibility to tetracycline and, therefore, may be selected for therapy in areas where such strains are known or suspected to exist. However, there is little clinical experience, as well as few in vitro susceptibility data, to support this choice. Azithromycin may also be used to treat pregnant woman infected with typhus.
A randomized trial in northern Thailand compared the efficacy of doxycycline alone with the combination of doxycycline and rifampin for scrub typhus. The median duration of fever was significantly shorter in patients treated with the combined regimen.
Prevention
Although there is no vaccine available to prevent the transmission of scrub typhus, several studies have demonstrated that doxycycline is highly effective for prophylaxis when used by nonimmune persons living and working in areas where scrub typhus is highly endemic. A weekly dose of 200 mg of doxycy-cline has been shown to be reasonably effective in preventing transmission.
Human Monocytic Ehrlichiosis
Epidemiology
The principal vector of HME is the Lone Star tick (Amblyomma americanum) The causative agent of HME, E. chaffeensis, was first isolated from a soldier at Fort Chaffee, Arkansas, in 1990. Since then, HME has been recognized as endemic throughout the southeastern and south central United States. In addition, a few cases of HME have been recognized in New England and the Pacific Northwest, and isolated cases have been reported in Europe, Africa, and Mexico.32
In some locations, HME is probably more common than RMSF. In a prospective study of 35 consecutive patients from North Carolina who presented to outpatient facilities with fever and a recent history of tick bite, 26% had HME, 17% had RMSF, and one patient was coinfected with R. rickettsii and E. chaffeensis. Most of these patients received outpatient treatment with doxy-cycline, and all of them recovered quickly.33
Estimates of the incidence of HME have varied widely. A prospective study of febrile patients hospitalized in southeast Georgia in the United States showed that the prevalence of HME was 5.7 cases per 100,000 population.34 A remarkably higher incidence was described in a golf-oriented retirement community in Tennessee, in which the annual incidence of disease was 660 per 100,000.® A serosurvey in the same community revealed that 12.5% of the residents had serologic evidence of past infection. Tick bites, exposure to wildlife, and golfing were associated with an increased risk of infection.
White-tailed deer are thought to be the principal animal reservoirs for E. chaffeensis infection. A study from Georgia found serologic evidence of E. chaffeensis infection in 27 of 35 deer and isolated E. chaffeensis from five of them, confirming that deer are naturally infected in endemic areas.36 Although the role of wild canids and domestic dogs in the epidemiology of HME is uncertain, in one study 15 of 21 coyotes trapped in Oklahoma were found to be infected with E. chaffeensis?7
Diagnosis
As with other tick-borne diseases, the diagnosis of HME is usually based on the recognition and synthesis of characteristic clinical and laboratory features in patients who reside in a geographic location where ehrlichiosis is known to occur during a time of year when tick exposure is likely or known. In other words, a history of tick bite or tick exposure during the spring or summer months in a resident of an endemic area, coupled with the presence of leukopenia or thrombocytopenia and abnormal liver function test results, provides strong circumstantial evidence for the diagnosis of HME. Even the presence of some of these findings is sufficient justification for the initiation of therapy.
Clinical Manifestations
After an incubation period of 5 to 14 days, patients with HME typically develop fever along with malaise and headache. Chills occur in approximately two thirds of patients, and gastrointestinal symptoms, including nausea and vomiting, may occur in up to one half of patients. Cough may also be a prominent symptom of HME, leading to diagnostic confusion with a host of respiratory illnesses. Although HME usually presents as an acute illness, subacute infection from E. chaffeensis has been described. In one study, for example, six of 41 patients with HME diagnosed at a medical center in Missouri during a 4-year period had protracted fever, ranging in duration from 17 to 51 days.38 In addition, rare cases of subclinical or self-limiting infection with E. chaffeensis have been described.
Skin rash is uncommon in patients with HME, but when present, it may be macular, maculopapular, or petechial. Although skin rash was reported in 36% of cases in one case series of 211 patients with HME, skin rash has been less common in the experience of many clinicians working in HME-endemic regions.39 The clinical features of HME are highly variable. Some patients present only with headache, anorexia, and malaise, whereas other patients have prominent neurologic symptoms that may include mental-status changes and stiff neck.
Laboratory Studies
The most common laboratory abnormalities seen in patients with HME are leukopenia (often accompanied by a left shift), thrombocytopenia, and elevated levels of aminotransferases (transaminases), lactate dehydrogenase, and alkaline phos-phatase. Anemia and an elevated plasma creatinine concentration also may be seen. Later in the course of illness or during recovery, a striking atypical lymphocytosis may occur.
Abnormalities of the cerebrospinal fluid are common in patients in whom a lumbar puncture is performed because of neurologic symptoms. Lymphocytic pleocytosis and elevated CSF protein levels were found in 21 of 38 patients with E. chaffeensis infection in one study.
The detection of morulae in lymphocytes in smears of the peripheral blood or buffy coat can occasionally be useful and even diagnostic. Unfortunately, morulae are seen in only a small minority of patients with HME. Thus, such testing has a low sensitivity, even though the finding of morulae is highly specific.
HME can be confirmed serologically with an IFA test using E. chaffeensis as the test antigen. IFA tests can be obtained through all state health departments. The current case definition of HME used by the Centers for Disease Control and Prevention requires at least a fourfold rise or fall in IFA titer against E. chaffeensis between the acute stage and convalescent stage, with a minimum titer of 1:64.41 An important limitation of this test is that antibodies first become detectable 2 to 3 weeks after the onset of the illness. Thus, as with RMSF, serology is useful only in confirming infection, not in the decision to initiate therapy
Culture of Ehrlichia is extremely difficult. Only a few isolates of E. chaffeensis have been made from humans, and in such cases, detection required over 30 days of cultivation.42 PCR-based testing is available for the diagnosis of HME, but such testing is performed only in special laboratories and remains mostly a research tool.
Differential diagnosis
Ehrlichiosis may be easily confused with RMSF, a wide number of common viral illnesses (e.g., mononucleosis), thrombotic thrombocytopenic purpura, hematologic malignancy, cholangi-tis, the early phases of hepatitis A infection, and community-acquired pneumonia.
Treatment
The treatment of HME is the same as that of HGE (see below).
Prognosis
Estimated mortality for patients with HME has ranged from 2% to 5%. The available mortality data are limited and are based on small case series, which may overestimate the actual risk.
Human Granulocytic Ehrlichiosis (Anaplasmosis)
Epidemiology
First described in 1994 in patients from the north central United States, HGE is now known to occur in Wisconsin, Minnesota, Connecticut, New York, Massachusetts, California, and Florida, as well as in western Europe.43 A population-based surveillance study from northwestern Wisconsin reported an incidence of 9.5 HGE cases per 100,000 population in an area where the incidence of Lyme disease was 57 cases per 100,000 population.44 Epidemiologic serosurveys have demonstrated that 3% to 15% of asymptomatic persons living in endemic areas may have antibodies to the HGE agent.45
A. phagocytophilum, the causative agent of HGE, is primarily transmitted by Ixodes scapularis, the tick that is also the vector of Lyme disease and babesiosis. I. pacificus, the black-legged tick, is the primary vector of HGE in the western United States, and I. ricinis is the presumed vector in Europe.
Diagnosis
The diagnosis of HGE must sometimes be made on a circumstantial basis, by synthesis of the history and the clinical and epi-demiologic features of an individual case. Clinicians should consider HGE as a possible diagnosis in any patient with a nonspecific febrile illness who becomes ill in the spring or summer months, who inhabits or has visited an endemic region, or who has a history of recent tick bite or tick exposure. Laboratory studies can support, and sometimes confirm, the diagnosis. However, the absence of such findings—in particular, the failure to detect morulae in leukocytes—should not dissuade clinicians when the overall picture suggests HGE.
Clinical Manifestations
The incubation period (5 to 14 days) and clinical features of HGE are similar to those of HME. Most patients with HGE have nonspecific symptoms such as malaise, myalgia, headache, nausea, vomiting, arthralgias, and cough. In a study of 18 adults with HGE, symptoms appeared an average of 5.5 days after a tick bite was noted.
Clinically, HGE can range from mild to severe. Fatal HGE has been documented: a retrospective case study of 41 patients with laboratory-diagnosed HGE infection found a case-fatality rate of 4.9%.47 Many of the patients who died had secondary opportunistic infections, such as fungal pneumonia. However, other studies have estimated that the mortality for HGE may actually be less than 1%.43
Laboratory Studies
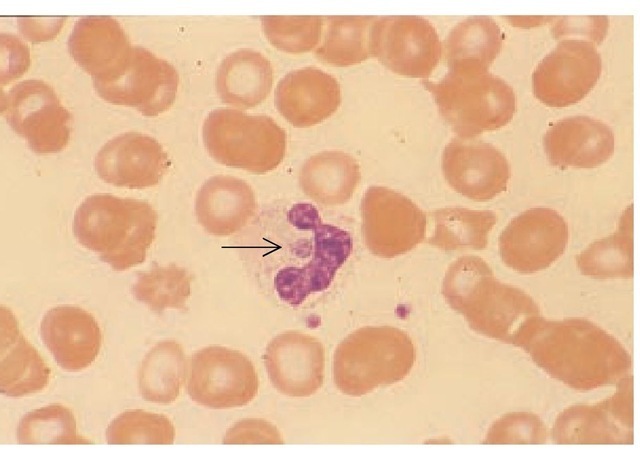
The diagnosis of HGE can be confirmed by finding characteristic intraleukocytic morulae in the peripheral blood [see Figure 4] or buffy coat, by serology (using IFA testing), or by PCR testing.
The frequency of detecting morulae in the peripheral smears of patients with HGE has varied in different case series. In one report, typical morulae were found in the peripheral smear in 28 of 35 patients with laboratory-confirmed HGE infection.47 The percentage of infected neutrophils ranged from 1% to 44% (me-dian, 5%). In another study of patients with HGE, from the upper Midwest and New York, morulae were visible in neu-trophils in 86 of 141 patients (61%).
Figure 4 In a peripheral blood smear from a patient with human granulocytic ehrlichiosis, a typical round ehrlichial morula is seen at the center of the neutrophil, adjacent to the nuclear lobes (arrow). Two platelets can be seen below and to the right of the neutrophil, for comparison. Wright-Giemsa stain was used; original magnification: x 370.
The most characteristic laboratory abnormality in patients with HGE is thrombocytopenia. In addition, patients with HGE typically manifest relative and absolute lymphopenia during the early phases of their infection, and significant increases in the band neutrophil counts occur during the first week of illness.48 It is important to emphasize that automated blood counts are unable to distinguish between band and normal neutrophils or to detect morulae. Therefore, a manual differential WBC should be ordered whenever HGE is suspected.
Diagnosis of Coinfection with Borrelia burgdorferi
Because the agent of Lyme disease and HGE are transmitted by the same vector, it is not surprising that a number of reports have described patients who were coinfected with B. burgdorferi and A. phagocytophilum.49 In studies of I. scapularis ticks from different locales, 2.2% to 26% of ticks were infected with both pathogens.50 The diagnosis of HGE can easily be missed in such patients because the typical rash of early Lyme disease (erythema migrans) may mislead the clinician into failing to consider the possibility of coinfection with A. phagocytophilum. Such coin-fection should be suspected when patients with presumed Lyme disease also have some or many of the following findings: leukopenia, thrombocytopenia, cough, high fever, or abnormal liver enzyme test results. Similarly, when rash is present in a patient with known or presumed HGE, the possibility of coinfec-tion with B. burgdorferi should be suspected.
Treatment
As with HME, treatment should be initiated in all patients suspected of having HGE. The drug of choice is doxycycline, given orally or intravenously at a dosage of 200 mg/day in two divided doses. Intravenous therapy is preferred for patients who are seriously ill or experiencing nausea or vomiting. Children should be given doxycycline at a dosage of 2.5 to 3 mg/kg/day in two divided doses. There is no consensus on the use of chlor-amphenicol; many experts advise against its use for any form of ehrlichiosis. Patients who have intolerance or allergy to tetracy-clines can be treated with rifampin for 7 to 10 days, but such patients require careful follow-up and monitoring, because there is only anecdotal information on the efficacy of this therapy in patients with HGE (and even less information on its efficacy in HME).43
At present, there are no guidelines for the treatment of ehrli-chiosis in pregnancy. However, a report describing the successful treatment of two cases of HGE using rifampin suggests that other Ehrlichia species (e.g., E. chaffeensis) may be susceptible to rifampin as well.51
In one study, A. phagocytophilum was susceptible in vitro to fluoroquinolones and rifampin. However, the most active fluo-roquinolone was trovafloxacin, which is not in general use because of concerns about hepatic toxicity.52
Even without treatment, most patients with ehrlichiosis probably make a full recovery. There is no evidence that untreated ehrlichiosis produces a chronic illness such as that which occurs with Lyme disease. However, infection may not confer long-lasting immunity. One report describes a patient who experienced two episodes of HGE spaced 2 years apart. At the onset of the second infection, the antibody titer to A. phagocytophilum had fallen from 1,280 to 80.53
Q Fever
Epidemiology and etiology
Q fever (the Q stands for "query") was first described in 1935 by Edward Derrick, after he investigated an outbreak of febrile illness involving abattoir workers in Queensland, Australia. Q fever is now known to be a worldwide zoonosis with highly variable clinical features. The causative organism, Coxiella burnetii, is a strictly intracellular pathogen that replicates and persists in cells in phagolysosomes. Microbial products, including acid phos-phatase, help the organism resist the acidic and presumably harsh vacuolar environment.54 In addition, vegetative C. burnetii cells form endogenous sporelike structures that resist extreme environmental conditions; spore formation has been observed in infected cardiac valves.55 Because of these microbiologic characteristics, C. burnetii is capable of surviving prolonged drying in dust and excreta and can remain viable for months in water and milk. C. burnetii can be grown in cultured mammalian cells and in small animals but is not able to replicate on cell-free media.
The most common reservoirs for C. burnetii are cattle, goats, and sheep. Animals infected with C. burnetii are rarely symptomatic, but they shed organisms in their milk, urine, feces, and placentas. Infected placental tissue, postpartum discharges, and fe-ces are presumed to be the principal sources of transmission to other animals and to humans. Domestic cats and dogs may also acquire C. burnetii and become sources for zoonotic transmission to humans.56 Numerous tick species are also naturally infected with C. burnetii. Such infected ticks may transmit C. burnetii to other generations of ticks transovarially and via tick bites to wild rodents. Livestock sometimes acquire C. burnetii infections from infected ticks, but most often, they become infected by inhalation of contaminated dust.
Dairy and slaughterhouse workers are at increased risk for acquiring Q fever. However, sporadic cases of Q fever may occur in humans who acquire infection via infectious aerosols that were generated at relatively long distances from the site of acquisition. Infected milk may also account for some outbreaks of the disease in humans. Human infection from tick bites is extremely rare. C. burnetii is highly infectious in the laboratory, and outbreaks have occurred in researchers. Transmission between humans is rare, but transmission during delivery (to an obstetri-cian)57 and sexual transmission58 have been described. Exposure to wild rabbits, parturient cats, and products of feline parturition may lead to Q fever pneumonia in humans, sometimes by an indirect route (e.g., from contaminated clothing). Such cases illustrate an important clinical point: Q fever may occur in urban dwellers without obvious or direct contact with animals.
Q fever is endemic throughout the six populated continents, but it is particularly common in the Mediterranean and Persian Gulf regions. The diagnosis of Q fever should be considered in travelers and military personnel who return to the United States from endemic areas. In the United States and Canada, Q fever still occurs sporadically, particularly in areas where cattle, sheep, and goats are raised. Because of the difficulty in diagnosis and because spontaneous improvement occurs in many patients who do not receive effective antimicrobial therapy, the true prevalence of Q fever in the United States is unknown.
Diagnosis
Clinical Manifestations
Q fever is usually classified into acute and chronic forms. Asymptomatic infection is also common. Acute Q fever may manifest as a self-limiting febrile illness, an influenzalike lower respiratory tract infection, hepatitis, or pneumonia. A small number of well-documented cases of meningoencephalitis from C. burnetii infection have also been described.59 Patients with chronic C. burnetii infection may develop granulomatous hepatitis with prolonged fever, myocarditis, pericarditis, or endocarditis.
The incubation period of Q fever ranges from 10 to 39 days, with an average duration of 20 days.60 The initial manifestations of acute C. burnetii infection are systemic and nonspecific— headache, chills, fever, myalgias, anorexia, and malaise. Skin rash does not occur in patients with Q fever. High fevers and headache often persist, and after 4 or 5 days of fever, patients typically manifest pneumonia, cough, chest pain, and inspirato-ry rales. In most cases, chest radiographs show focal areas of pneumonitis. Radiologic changes may be more marked than symptoms or physical findings. A minority of patients with Q fever pneumonia develop pleural effusions, hemoptysis, and even respiratory failure.61 Pulmonary involvement occurs in about 50% of patients, but the incidence of this complication may vary with geographic location. In certain parts of the world, hepatitis may be more common than pneumonia in patients with acute Q fever.62 Hepatitis in patients with acute Q fever usually lasts for 1 to 2 weeks and follows a benign, self-limited course. Complications are rare. However, granulomatous hepatitis with hepatosplenomegaly, jaundice, and abnormal liver function test results can also persist along with fever for up to 3 or 4 weeks.
In approximately 2% to 11% of infected persons, a chronic form of Q fever will develop insidiously a few months to as long as 20 years after the acute illness. Risk factors for chronic Q fever include underlying valvular heart disease and an immunocom-promised state.63 Infective endocarditis caused by C. burnetii is a common feature of the chronic syndrome.64 The disease may affect healthy valves, previously damaged native valves, or prosthetic valves. Q fever endocarditis is sometimes insidious in onset and is often accompanied by granulomatous hepatitis. Other forms of endovascular infection with C. burnetii also occur, including infections of aneurysms and grafts.
Laboratory Studies
The diagnosis of acute Q fever is usually established by demonstration of a fourfold or greater rise in complement-fixing antibody titer against C. burnetii phase II antigen.65,66 IFA techniques and ELISA offer greater sensitivity than complement-fixation methods. IFA techniques for the early detection of specific IgM antibody are the serodiagnostic method of choice. The diagnosis of chronic Q fever or Q fever endocarditis is established by detecting elevated titers (i.e., > 1:200) of IgG or IgA antibodies against C. burnetii phase I antigen or by a ratio of anti-phase I antibody to anti-phase II antibody of 1 or greater.
C. burnetii can be isolated from blood, sputum, or urine by in-traperitoneal inoculation in guinea pigs, inoculation into chick embryos, or inoculation of cultured human fetal diploid fibro-blasts. Because this organism is so highly contagious in the laboratory, attempts at isolation should be made only in a special biologic containment facility.
Echocardiography may not be diagnostic in Q fever endocarditis. A study that examined the heart valves removed from 28 patients with Q fever endocarditis showed that infected valves were usually fibrotic and calcified but that most had only slight inflammation. Only two of 16 patients with native Q fever endocarditis and two of 10 patients with bioprosthetic Q fever endocarditis had macroscopic vegetations, yet C. burnetii was isolated by culture in 64% of these patients and identified by PCR in 75%.67
Differential diagnosis
Early in its course, Q fever resembles a variety of acute febrile illnesses, including an array of viral respiratory infections, viral hepatitis, and infectious mononucleosis. In patients with a history of contact with livestock, other zoonoses (e.g., brucellosis and leptospirosis) should be considered along with Q fever. In patients who present with pneumonia, infection with Mycoplasma pneumoniae, Chlamydia pneumoniae and C. psittaci, Legionella pneumophila, Histoplasma capsulatum, and the agents causing viral pneumonia should also be considered in the differential diagnosis. When hepatitis is present and noncaseating granulomas are found on liver biopsy, a host of causes of granulomatous hepatitis (e.g., tuberculosis, sarcoidosis, brucellosis, and histoplasmo-sis) must be considered. Q fever should always be considered when the clinical features of endocarditis are present but blood cultures are negative.
Treatment and prevention
Effective treatment for acute Q fever is tetracycline, 500 mg orally every 6 hours; doxycycline, 100 mg orally every 12 hours; or ciprofloxacin, 500 mg orally every 12 hours. Most patients treated early in the course of the infection recover rapidly; however, acute Q fever is usually self-limited.
If left untreated, Q fever endocarditis is fatal. Valve replacement is often necessary, in addition to prolonged antimicrobial therapy. Prolonged antibiotic therapy is necessary both for those who have valve surgery and for those in whom medical therapy alone is used. A study of cardiac valves in patients with Q fever endocarditis found that histologic, microbiologic, or molecular detection of C. burnetii was possible in more than 80% of patients who had been treated for less than 1 year; cultures and PCR tests were still positive in 22% and 33% of patients, respectively, who were treated for more than 1 year before valve excision.67
Medical regimens employing different antimicrobial agents, either alone or in combination, have been tried with variable success in Q fever endocarditis.68,69 Some experts recommend treatment with doxycycline plus rifampin or with doxycycline plus a fluoroquinolone for at least 3 years.70 Chloroquine has been also used as adjunctive therapy because of its ability to block intracel-lular vacuole acidification and, hence, growth of C. burnetii.
Patients with Q fever need not be isolated, because secondary cases do not occur. Killed vaccines made from C. burnetii grown in chick embryo cultures are immunogenic and can provide protection for persons at high risk, such as dairy and slaughterhouse workers, woolsorters, tanners, and laboratory workers. However, vaccines against C. burnetii are not commercially available for human use in the United States.