Genetic Counseling and Prenatal Diagnosis
Parents who have had a stillborn hydropic infant or a child with Cooley anemia are justifiably reluctant to repeat the experience. Adults from thalassemia families who know themselves to be heterozygous for thalassemia are often eager to receive genetic counseling when starting their own families. Genetic counseling entails screening prospective parents on the basis of routine diagnostic tests and family studies. In addition, advances in molecular genetics can now provide accurate, unambiguous prenatal diagnoses of the thalassemias. In the first trimester, chorionic villus sampling combined with the use of polymorphic DNA markers and synthetic oligonucleotide probes can provide the definitive diagnosis in about 80% of cases of | -thalassemia [see 9:VII Basic Genetics for the Clinician].127,128 Indeed, the incidence of births of infants with thalassemia major has fallen in several parts of the world. Different ethnic groups respond differently to genetic counseling.
Extracorpuscular Defects
Erythrocytes can be damaged through trauma or by antibodies, drugs, abnormally functioning organs, and toxins. These causes of an extracorpuscular defect should be considered whenever hemolysis develops in a patient who has no personal or family history of anemia.
Mechanical mjury: microangiopathic hemolysis
Microangiopathic hemolysis is characterized by the appearance of bizarre, fragmented erythrocytes (e.g., schistocytes, or helmet cells) on a peripheral smear and by signs of intravascular and extravascular hemolysis.
Pathophysiology
The normal erythrocyte can withstand considerable elongation and twisting, but it disintegrates when subjected to strong stretching or shearing forces. Stresses of this magnitude have been observed to occur in jets produced by deformed aortic valves, by arteriovenous shunts, by ventricular septal defects, or by the older valvular prostheses.
Localized intravascular coagulation, in which fibrin strands bridge the arteriolar lumen, is thought to occur in arterioles supplying inflamed or neoplastic tissues. Fibrin strands lop off fragments of RBCs, whose membranes promptly reseal. Some of the erythrocyte contents leak out, however, producing varying degrees of intravascular hemolysis. The distorted RBCs are then removed by the reticuloendothelial system.
Diagnosis
Hemolysis in conjunction with typical blood smear findings is diagnostic of microangiopathic hemolysis [see Figure 5]. If the angiopathy is extensive, thrombocytopenia and disseminated in-travascular coagulation develop. Causes include hemodynamic jets, vasculitis,129 giant hemangiomas, thrombotic thrombocyto-penic purpura, metastatic cancer,130 certain infections (especially meningococcemia, rickettsial diseases, and hantavirus infection), hemolytic-uremic syndrome, disseminated intravascular coagulation, drugs (cocaine, cyclosporine, mitomycin, and tacrolimus), and even subclavian catheters.130,131 Quinine has been identified as a fairly common cause of drug-induced thrombotic thrombo-cytopenic purpura (TTP) and hemolytic-uremic syndrome.132 A single case of microangiopathic hemolysis has been described in an infant with cutaneous anthrax.
Treatment
In treating microangiopathic hemolysis, clinicians must focus primary attention on the underlying disease. Patients may become iron deficient and require iron therapy. Supplementation of depleted folate stores may stimulate erythropoiesis. In rare cases, anemia caused by an old prosthetic aortic valve may be severe enough to warrant valve replacement. Plasmapheresis provides effective therapy for TTP [see 5:X Transfusion Therapy and 5:XIIIHemorrhagic Disorders].
March hemoglobinuria March hemoglobinuria, a disorder that somewhat resembles microangiopathic hemolysis, usually occurs in young persons after prolonged marching or running or playing on bongo drums. The severe and repetitive trauma to the feet or hands is thought to destroy RBCs circulating in the vessels of the soles and palms. The patient notices red urine that clears in 1 day or less after the activity. Transient hemoglobine-mia and hemoglobinuria without anemia, smear abnormalities, or reticulocytosis confirm the diagnosis. The use of padded shoes and the avoidance of paved surfaces may prevent recurrences in persons who continue running.
Immune hemolysis
General Mechanisms
A classic, well-delineated example of immune (not autoimmune) hemolysis involves fetomaternal incompatibility at Rh locus D, in which the D-negative mother, after contact with D-posi-tive erythrocytes, may produce an IgG anti-D antibody; the antibody crosses the placenta and attacks and destroys fetal erythrocytes. The fetus becomes jaundiced and has spherocytic erythrocytes.
The fetal RBCs, now coated with maternal IgG anti-D antibody, attach to fetal macrophages and monocytes that contain receptors for the Fc portion of these IgG molecules. Macrophagic digestion of portions of the erythrocytic membrane leads to the loss of considerable surface area. The resulting rigid spherocyte returns to the circulation and becomes trapped in the fetal reticu-loendothelial system, particularly in the spleen. Hemolysis results. The IgG antibody is maximally active at 37° C; it generally cannot extensively activate the complement pathway, and it cannot agglutinate attacked RBCs suspended in saline.
The direct Coombs antiglobulin test [see Figure 7] is used clinically to detect IgG coating of RBCs. This test is negative in the mother, because her erythrocytes lack D antigen and thus are not coated with anti-D antibody. The indirect Coombs test [see Figure 7], which detects the presence of free serum antibody that reacts with RBCs, is positive for the mother’s serum because she has circulating anti-D antibody. In the fetus, in contrast, the direct Coombs test is strongly positive because the fetus’s RBCs, which express D antigen, are coated with maternal anti-D antibody. The results of the fetus’s indirect Coombs test may be positive or negative, depending on the amount of anti-D antibody that has been transferred by the mother, the avidity of the anti-D antibody for fetal D-positive RBCs, and the availability of D antigen sites on fetal RBCs.
These antibodies are described as warm (maximum activity at 37° C [usually IgG1 or IgG3]) or cold (maximum activity at 5° C [usually IgM]). Antibodies have also been classified as complete (i.e., capable of agglutinating saline-suspended RBCs) and incomplete (i.e., incapable of agglutinating saline-suspended RBCs); their detection requires the use of techniques such as the direct Coombs antiglobulin test [see Figure 7] or enzyme treatment of RBCs.134 Warm autoantibodies are usually incomplete, whereas cold agglutinins, which are for the most part IgM, are usually complete.
Autoimmune Hemolytic Anemia
Autoimmune hemolytic anemia is generally an acute disorder characterized by extravascular hemolysis. Intravascular hemoly-sis in this condition is rare and indicates that an extremely rapid rate of erythrocyte destruction is occurring or that the extravas-cular removal mechanisms have been overwhelmed.
Pathophysiology In autoimmune hemolytic anemia, for reasons that are unclear, autoantibodies form and are directed against central components of the erythrocyte (e.g., Rh antigen, Kell antigen,134 glycophorin A).136 Alternatively, the patient’s RBCs are sensitized with both an IgG antibody and a complement component, usually C3d. In other circumstances, however, it appears that complement is fixed to the RBC surface by an IgM antibody that is subsequently washed away. Occasionally, the RBCs exhibit only complement components, and no IgG can be detected by the Coombs test. Complement fixation in such cases may be explained by the continued presence of IgG at a level below that detectable in the usual direct antiglobulin test; alternatively, a complement-fixing IgG or IgM antibody had been attached to the cell but was eluted in the testing procedure.
The severity of hemolysis correlates with the number and class of IgG and, in rare cases, IgA molecules attached to the RBC surface. Antibody-coated RBCs attach to the macrophages’ receptors (FcRI, FcRII, or FcRIII) by the antibody’s Fc portion. The firm binding of RBCs to these macrophage receptors is then followed either by removal of a portion of the RBC membrane, which results in the production of a spherocyte, or by phagocytosis of the entire RBC.134 Relatively low levels of IgG1 attachment to RBCs produce a positive result on direct Coombs antiglobulin testing without evidence of hemolysis (approximately 1,000 molecules per RBC), whereas much higher levels of IgG1 autoantibody per RBC are associated with frank hemoly-sis.134 The combined presence of IgG and complement components may enhance the severity of hemolysis.
Erythrocytes sensitized to IgG alone are usually removed in the spleen, whereas RBCs sensitized to IgG and complement or to complement alone are generally destroyed in the liver, because hepatic Kupffer cells carry receptors specific for complement component C3b.
Differential diagnosis Both an idiopathic variety of autoimmune hemolytic anemia and a variety that occurs secondary to other disorders have been described. Such primary disorders include systemic lupus erythematosus, non-Hodgkin lymphoma (especially chronic lymphocytic leukemia), Hodgkin disease, cancer, myeloma, dermoid cyst, HIV infection, angioimmunoblas-tic lymphadenopathy with dysproteinemia, hepatitis C,137 and chronic ulcerative colitis.
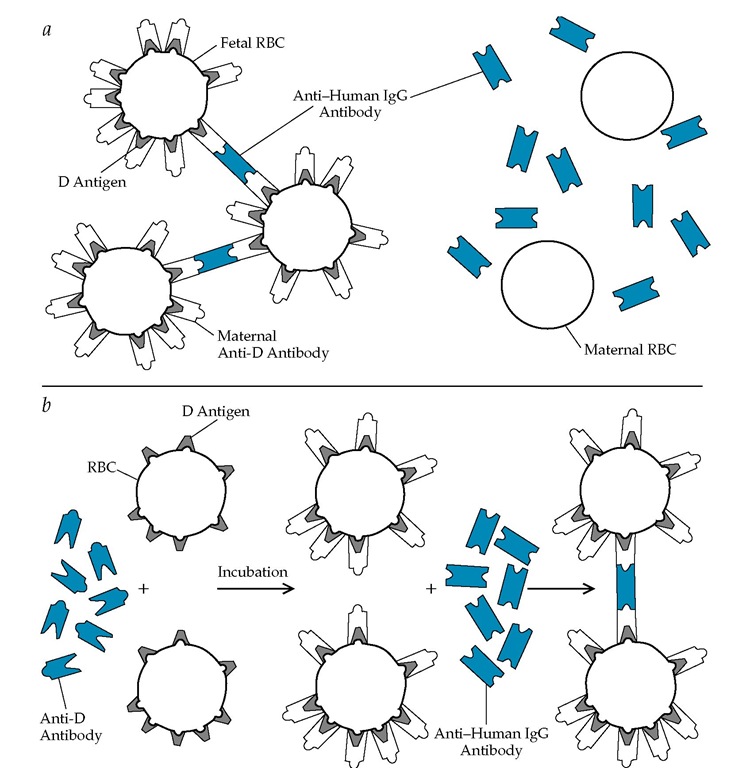
Figure 7 The Coombs test detects the presence of human antibodies or complement components on erythrocytes or the presence of antibodies in serum. The test is useful in diagnosing Rh hemolytic disease of the newborn, autoimmune hemolysis, or potential hemolytic transfusion reactions. The figure illustrates Rh hemolytic disease of the newborn.
In the direct Coombs test (a), fetal erythrocytes (RBCs) are shown with D antigen attached to their surfaces. Maternal anti-D antibody binds to the fetal erythrocytes at the D antigen sites in utero. Coombs antiserum, which contains antibody to human IgG, binds to the anti-D antibody on a sample of washed fetal erythrocytes, causing them to clump (a positive reaction). Washed maternal erythrocytes, having no D antigen, will have no attached anti-D antibody and are therefore not clumped by Coombs serum (a negative reaction).
In the indirect Coombs test (b), maternal or fetal serum is added to the red cells of another person or to panels of erythrocytes of known antigenic specificity; Coombs antiserum is then added. Clumping in this case occurs only if the test serum, such as the maternal serum, contains anti-D antibody and if the red cells chosen have the D antigen.
The direct Coombs test is used to detect immunoglobulin molecules already attached to erythrocytes, such as those found on the fetal erythrocytes in Rh hemolytic disease of the newborn or in autoimmune hemolytic anemia. Therefore, the test is done on the patient’s thoroughly washed erythrocytes. The indirect Coombs test is used for determining whether specific antibodies are present in a serum sample, and it is performed on the patient’s serum.
In severe cases, the blood smear shows macrocytosis, poly-chromatophilia, variable spherocytosis, and autoagglutination of RBCs. The platelet count is also occasionally depressed (Evans syndrome), and there may be leukopenia. One third of patients may have reticulocytopenia at presentation.138 The direct Coombs test will be positive. Any or all of these findings may be absent in mild disease.
Whether complement, IgG, or both are present on RBCs should be determined by the use of Coombs reagents that are specifically directed against IgG, IgA, or complement components. Occasionally, an autoimmune hemolytic anemia is suspected, but the direct Coombs test is repeatedly negative; in such cases, the level of autoantibody may be below the level of de-tectability for very active autoantibodies, such as subclass IgG3 autoantibodies, or the autoantibody may be IgA or IgM.
Patients with evidence of hemolytic anemia should be screened for autoimmune diseases (e.g., systemic lupus erythe-matosus) and other forms of hemolysis, such as paroxysmal nocturnal hemoglobinuria, cold agglutinin disease, and paroxysmal cold hemoglobinuria.
Treatment Treatment of clinically affected patients is directed at decreasing autoantibody production and reducing the macro-phagic attack on the RBCs. Initial therapy usually consists of 60 to 100 mg of prednisone a day, given in divided doses. This approach usually produces a slow decrease in antibody coating of RBCs and is thought to interfere with phagocytic attack on coated erythrocytes. A good response to corticosteroid use—indicated by a rise in the reticulocyte count and an improvement in hemoglobin and hematocrit—may be apparent within 1 or 2 days. Supplementation with 1 mg of folic acid a day is recommended.
After the initial response to therapy, which is usually satisfactory, the hemoglobin level and reticulocyte count may return to normal. The Coombs test is then repeated to determine whether the response has become weaker; if so, the prednisone dosage is tapered cautiously. Approximately 20% of patients remain well indefinitely, but the majority suffer from a chronic, treacherous disease that can produce sudden relapses with abrupt anemia. The prednisone should be titrated in accordance with the hemoglobin level, the reticulocyte count (elevation indicates continued hemolysis), and the direct Coombs titer; alternate-day therapy should be considered to minimize steroid side effects. If patients do not respond to standard prednisone therapy, high-dose dex-amethasone (e.g., 40 mg/day orally for 4 consecutive days in 28-day cycles139) may be effective.
If the corticosteroid dose required for long-term therapy produces significant morbidity, one can proceed empirically either to splenectomy or to the use of immunosuppressive agents. Measurements of splenic sequestration of chromium-51 (51Cr)-la-beled erythrocytes do not reliably indicate the benefits of splenectomy. Splenectomy rarely results in extended remission but is valuable as a prednisone-sparing measure. After splenec-tomy, low-dose prednisone (5 to 10 mg/day) may stabilize the hemoglobin concentration.
The immunosuppressive agent azathioprine or cyclophos-phamide can be used as an alternative to splenectomy. There is no reliable evidence to support the use of one of these agents over the other. For patients with very aggressive disease, cy-closporine has been used successfully.140 Azathioprine should be started at a dosage of 100 to 200 mg a day; the peripheral blood count should be monitored with a view toward preventing retic-ulocytopenia or neutropenia. Cyclophosphamide is started at a dosage of 100 to 200 mg a day, with monitoring of blood counts and urine; however, because cyclophosphamide can cause therapy-related acute myeloid leukemia or myelodysplastic syndrome, its use should be limited [see 12:XVII Chronic Myelogenous Leukemia and Other Myeloproliferative Disorders].
Azathioprine or cyclophosphamide doses have to be adjusted to reduce the white cell count to about 3,000/mm3. Improvement usually comes in 3 to 4 weeks. When a response occurs, the pred-nisone dose can be reduced and the hemoglobin level, reticulo-cyte count, and Coombs titer monitored to determine the minimally required therapy. For patients with very refractory disease, therapy with intravenous cyclophosphamide at doses used for allogeneic bone marrow transplantation has been tried. This approach is clearly myelotoxic, and its usefulness awaits further confirmation. High-dose intravenous IgG has been used to treat autoimmune hemolytic anemia. In one report, only one third of patients had a transient response, and doses larger than those used in idiopathic thrombocytopenic purpura (i.e., 1g/kg/day for 5 days) were required.141,142
There are anecdotal reports that rituximab is useful in treating refractory and relapsing cases.143,144 However, a case of automim-mune hemolytic anemia occurring after rituximab therapy for lymphoproliferative disorder has been reported.
Patients with symptomatic anemia require an RBC transfusion, but often the blood bank reports an incompatibility. Many blood banks regularly perform a direct Coombs antiglobulin test on the recipient’s RBCs. A patient who has free antibodies in the serum will exhibit very extensive and broad reactivity against donor panels of RBCs and will usually produce an incompatible major cross-match when tested with the antiglobulin reagent. If transfusions are needed to support cardiorespiratory and central nervous system functions, immediate consultation with the transfusion medicine service is recommended.135 No patient should be allowed to die because the blood bank does not have a perfectly compatible unit of RBCs. If transfusion is clinically indicated, the physician should administer the best units of blood that are available, because it has been shown that these patients can tolerate even imperfectly matched RBCs.146
Drug-Related Immune Hemolysis
Drug-initiated immune hemolysis is often indistinguishable from autoimmune hemolytic anemia. There are two variants: the hapten type and the hemolysis that results from alteration of a membrane antigen.
Hapten type Drugs such as the penicillins and the cepha-losporins bind firmly to the erythrocyte membrane. In rare circumstances in which massive dosages of the drug (e.g., more than 10 million units of penicillin a day) are required, the protein-bound drug may act as a hapten and elicit an immune response. An IgG antibody that appears to be directed against the drug-RBC complex is produced148,149; this leads to a positive result on direct Coombs testing with the anti-IgG reagent and a negative result with the anti-C3d reagent. When the offending drug is stopped, hemolysis ends in a few days. In contrast, the drug may be bound loosely to produce a neoantigen that generates the immune response.147 In this circumstance, the result of direct Coombs testing with the anti-C3d reagent is usually positive, and the result of testing with the anti-IgG reagent may be negative.
If the patient’s serum is tested against normal cells (i.e., the indirect Coombs test is used), no reaction occurs unless the offending drug and a source of complement are first added to the normal RBCs. Stopping or switching the drug is effective in eliminating the hemolysis because the antibody is usually very specific.
Alteration of a membrane antigen Some drugs may alter a membrane antigen, thereby stimulating the production of IgG antibodies that cross-react with the native antigen. Methyldopa is the classic example of a drug that causes autoimmune he-molytic anemia. Other examples are levodopa, mefenamic acid, and procainamide. Drug administration leads to a positive direct Coombs test with anti-IgG reagents in 15% to 20% of treated patients, but hemolysis occurs in fewer than 1%. The eluted antibody is seen to be a classic IgG autoantibody directed against Rh components. The mechanism of hemolysis is identical to that of autoimmune hemolytic anemia.
The NSAID diclofenac sodium has been reported to cause a devastating acute hemolytic anemia, with evidence of intravas-cular and extravascular hemolysis accompanied occasionally by shock, organ failure, and even disseminated intravascular coag-ulation.151 Patients develop both RBC autoantibodies and drug-dependent antibodies. It is thought that diclofenac sodium binds to the surface of RBCs, forming neoantigens that lead to the generation of true autoantibodies, as well as drug-dependent antibodies. The direct Coombs test is positive with both the IgG and the C3d reagents. Additional antibody reactivity occurs with the addition of diclofenac sodium metabolites obtained from the urine of patients treated with the drug. Therapy consists of recognizing the cause, stopping the diclofenac sodium, and supporting the patient for several days until the process stops.151
Delayed Hemolysis of Transfused Erythrocytes
Blood is usually typed only for ABO and Rh-D antigens, but other antigens are also present on RBCs. Thus, a patient who receives extensive transfusions over 1 to 2 weeks may develop an antibody response to one or more of these other antigens. Kell, Duffy, Kidd, and Rh antigens other than D are the usual offenders. When the patient with antibodies receives RBCs expressing these antigens, an acute self-limited hemolysis, usually extravas-cular, may ensue. Clues are a history of transfusion, spherocyto-sis on peripheral smear, a positive direct Coombs test, and the recent appearance of an antibody in the patient’s serum (positive indirect Coombs test). Usually, no therapy is required, but further transfusions should be cross-matched with the patient’s serum [see 5:X Transfusion Therapy]. Similar problems arise with transplantation of bone marrow and other tissue.