Genetic Counseling
A key element to be considered in the provision of genetic counseling to patients with sickle trait or sickle disease is the significant morbidity in affected children and adults. Couples with sickle disease or sickle trait may want to have children despite the associated fetal and maternal risks. There are about 4,000 to 5,000 such pregnancies in the United States each year.89 In one study, 286 of 445 pregnancies (64%) in mothers with sickle cell anemia proceeded to delivery; 21% of the infants were small and thus would be expected to require additional care, which the mother might have difficulty providing. In this study, there was one maternal death caused by sickle cell disease90 [see Genetic Counseling and Prenatal Diagnosis, below].
Prognosis in Sickle Cell Disease
Whereas it was once assumed that most patients with sickle cell anemia would die by 20 years of age, the median age of death is now 42 years for men and 48 years for women.51 This life expectancy is 25 to 30 years less than that of the general African-American population. Of the identified causes of death, only 18% involved organ failure—predominantly renal disease, heart failure, or the consequences of chronic strokes. Thirty-three percent of patients died during acute pain crises; these crises were frequently associated with the acute chest syndrome and were less often associated with stroke. The presence of a-thalassemia had no measurable effect. Predictors of poor outcome were a white cell count greater than 15,000/ M; a low HbF level; and organ involvement manifested by renal disease, acute chest syndrome, and neurologic events. Taking hydroxyurea had a significant impact on prognosis, with a 40% decrease in mortality and a reduction in painful crises.
Sickle Variants
Sickle Trait
Heterozygosity for the sickle cell gene results in sickle trait (HbAS). The RBCs of persons with sickle trait have an HbS concentration of less than 50%; frequently, the level is as low as 30%.
Generally, persons with sickle trait lead normal, healthy lives. A few complications occur: hyposthenuria; renal hematuria; and, during pregnancy, bacteriuria and pyelonephritis. Splenic infarction occurs under conditions of hypoxia; it also occurs at high altitudes, predominantly in nonblack persons who have sickle cell anemia.
Sickle trait has been identified as a major risk factor for sudden death during basic training in the military92; death has resulted from unexplained cardiac arrest, heatstroke, heat stress, or rhabdomyolysis. Increasing age has been correlated with an increased risk of sudden death. However, these events have occurred under extreme conditions: very strenuous physical activity, usually in untrained persons, occasionally at high altitudes or in extreme heat. Usually, persons with sickle trait who are accustomed to physical activity do not have an increased risk of sudden death. For example, the incidence of sudden death in African-American football players with sickle trait is not higher than in other players.93
Therapeutic options for renal hematuria include the administration of diuretics, parenteral bicarbonate, transfusions, or e-aminocaproic acid.
Sickle Cell-ft-Thalassemia
When combined with sickle trait, a defect in the | -thalassemia gene produces a disease very similar to sickle cell anemia. The | -thalassemia gene reduces the rate of synthesis of the | A chain, resulting in a predominance of | S in patients with sickle trait. Depending on whether the patient has a |0 or a |+thalassemia, the RBCs contain varying amounts of HbS, HbA, HbA2, and HbF. Patients with |0 thalassemia have no HbA, but only HbS, HbF, and HbA2; thus, disease is severe in these patients. Diagnosis is based on an elevation in the level of HbA2, HbF, or both on hemoglobin electrophoresis, as well as a positive family history of thalassemia and the sickle gene. In a study of 55 Greek patients, treatment with hydroxyurea resulted in distinct clinical im-provement.94,95 Further description and information on diagnostic testing is available online at http://www.geneclinics.org.
Sickle Cell-Hemoglobin C Disease
In sickle cell-hemoglobin C (HbSC) disease, almost equal amounts of HbS and HbC are formed. Between 1% and 2% of hemoglobin is HbF, and small amounts of HbA2 are also present; however, HbA is absent. The increased sickling seen in these patients results from the pathologic effect of HbC [see Hemoglobin C Disease, below].96 As many as 30% to 50% of patients with this disorder are not anemic and have only modest reticulocytosis. Patients may not be identified until the disorder manifests itself in the form of a vaso-occlusive crisis during surgery, pregnancy, or a medical emergency.97 Splenomegaly, proliferative retinopathy, aseptic necrosis of long bones, and the acute chest syndrome96 also occur. The peripheral smear [see Figure 5] shows irreversibly sickled cells in addition to target cells, stomatocytes, and erythrocytes with eccentric hemoglobin depositions, probably representing HbC aggregates or crystals. Diagnosis is confirmed by hemoglobin electrophoresis or HPLC.97 Further information on diagnostic testing is available online at http://www.geneclinics.org.
Management is the same as that for sickle cell anemia. In a study of six patients with HbSC disease, treatment with hydroxy-urea at a dosage of 1,000 mg/day resulted in an increase in MCV, a decrease in so-called stress reticulocytes, an increase in hemoglobin, and probably a reduction in cell density. Although not definitive, this small study suggests that hydroxyurea benefits patients with HbSC disease.98 Life expectancy for patients with HbSC disease is almost 20 years greater than that for patients with HbSS disease.51
Other Hemoglobinopathies
Hemoglobin C Disease
The relative insolubility of HbC is responsible for the pathologic changes associated with its presence. HbC probably interacts with the K+-Cl- cotransporter, which keeps it active, whereas the K+-Cl- cotransporter normally shuts off in RBCs after the reticulocyte stage. The result is a loss of K+, cellular dehydration with elevated MCHC, and then aggregation and crystallization of the poorly soluble HbC.97 The relative insolubility of HbC causes erythrocytes to become rigid and thereby subject to fragmentation and to loss of membrane material, resulting in the mi-crospherocytes seen on a peripheral blood smear [see Figure 3].
Target cells, an important morphologic finding, constitute about 80% of the erythrocytes. HbC crystals are in the oxyhemo-globin state and dissolve when the RBCs are deoxygenated, probably accounting for the absence of vaso-occlusive episodes.
Diagnosis of hemoglobin C disease is based on blood-smear findings and the absence of evidence of either iron deficiency or thalassemia; the diagnosis is confirmed by hemoglobin elec-trophoresis. No therapy is required.
Hemoglobin E Disease
In hemoglobin E disease, lysine is substituted for glutamic acid at position 26 of the | -globin chain, resulting in an oxida-tively unstable molecule. Hemoglobin E trait is found predominantly in Southeast Asia. It came to clinical attention in the United States as a result of the influx of Southeast Asians, in whom the incidence of this trait is about 10%.
Patients heterozygous for HbE have normal hemoglobin values, microcytosis, and no splenomegaly. Electrophoresis reveals that 70% of the hemoglobin is HbA, 25% is HbE, and the remainder is HbA2 or HbF. Inexperienced laboratories may mistake HbE for HbA2; the clue to this error is that HbA2 never accounts for more than 8% of the total hemoglobin. A laboratory report of an HbA2 level of 25% should prompt a review of the data.
Patients homozygous for HbE have mild anemia, with a hemoglobin level of about 12 to 13 g/dl, a low mean corpuscular volume, and an elevated RBC count but no reticulocytosis; they exhibit microcytes and target cells. Electrophoresis shows only HbE. Chronic hemolysis does not occur. Oxidant drugs such as dapsone should be avoided in both heterozygotes and homozygotes.
A serious clinical problem occurs when a patient is doubly heterozygous for HbE and | -thalassemia trait. Such patients present with ^-thalassemia intermedia, characterized by severe anemia and splenomegaly (see below). These patients occasionally require transfusions of blood and even allogeneic bone marrow trans-plantation.99 Further information on diagnostic testing is available online at http://www.geneclinics.org.
Unstable Hemoglobinopathies
Many individual variants make up the unstable hemoglo-binopathies. The hemoglobin instabilities stem from amino acid substitutions that deprive the molecule of its heme group, alter the heme pocket, loosen the link between its a and | chains, or weaken the subunit structure [see 5:I Approach to Hematologic Disorders]. The result is disruption and precipitation of hemoglobin, particularly when it is subjected to oxidant attack. Precipitated hemoglobin forms Heinz bodies, which are observed even in persons heterozygous for the unstable hemoglobin variant. Because of the deleterious effects of Heinz bodies on the erythro-cyte and its membrane, significant hemolysis can occur even in the heterozygous state.
Diagnosis of an unstable hemoglobinopathy is suggested by the presence of a partly compensated chronic nonspherocytic he-molysis. Heinz bodies are observed in the erythrocytes of patients who have undergone splenectomy. Erythrocytes from patients who have not undergone splenectomy demonstrate Heinz bodies on incubation with brilliant cresyl blue dye. The differential diagnosis of a hemoglobinopathy includes G6PD deficiency; this disorder can usually be ruled out by direct assay for the enzyme.
Management includes avoidance of oxidant drugs. Splenecto-my may be considered when hemolysis is severe and inadequately compensated.
Hemoglobin with Abnormal Oxygen Affinity
The presence of hemoglobin with increased oxygen affinity should be considered in the differential diagnosis of unexplained erythrocytosis, particularly if there is a familial association [see 5:V The Polycythemias]. Hemoglobin electrophoresis may reveal the disorder, but in suspected cases, measurement of the oxyhe-moglobin dissociation curve [see 5:I Approach to Hematologic Disorders] is preferable as a basis for diagnosis. Hemoglobin Chesapeake and hemoglobin Rainier are examples of forms with particularly increased oxygen affinity.
The rare instances of hemoglobin with low oxygen affinity, such as hemoglobin Kansas, represent mutations. Patients with low-oxygen-affinity hemoglobinopathy are sometimes cyanotic because of enhanced oxygen unloading.
Methemoglobinemia
Methemoglobin is an oxidation product of hemoglobin in which iron is in the ferric form; thus, the molecule cannot bind oxygen reversibly. Ordinarily, 1% of hemoglobin is in the ferric state. Between 0.5% and 3% of deoxyhemoglobin is normally spontaneously oxidized to methemoglobin every day. The normal reducing power of erythrocytes [see Table 1] maintains the balance between oxidation and reduction. The enzyme system that reduces 95% of methemoglobin to hemoglobin involves two proteins, NADH-cytochrome b5 reductase and cytochrome b5, and also requires NADH. As the name suggests, NADH-cy-tochrome b5 reductase uses NADH to reduce cytochrome b5. Reduced cytochrome b5 then reduces methemoglobin.100,101 Novel mutations in the affected gene have been described.102
Most often, methemoglobinemia is acquired by ingestion of or exposure to oxidants that oxidize Fe2+ so fast that the reducing systems are overwhelmed [see Mechanism of Oxidative Attack, below].
There are two congenital forms of methemoglobinemia. In the hereditary enzymopenic form of methemoglobinemia, patients are homozygous or doubly heterozygous for a deficiency of NADH-cytochrome b5 reductase.103 These patients appear blue even when only about 10% of their hemoglobin is in the form of methemoglobin, but they are not sick and easily tolerate methe-moglobin levels of 25% or more. In contrast, the presence of about 5 g/dl of reduced, deoxygenated hemoglobin produces cyanosis. Patients with this form of methemoglobinemia do not exhibit hemolysis and generally do not require treatment. Assay of NADH-cytochrome b5 reductase, done by a special laboratory, can establish the diagnosis. If desired, methylene blue at a dosage of 100 to 300 mg/day orally can be used, but it may produce urinary discomfort.100 Methylene blue transfers electrons from NADPH to methemoglobin.
The other hereditary form of methemoglobinemia is caused by HbM, of which there are five rare variants. Each of these variants contains an amino acid substitution in the heme pocket, which allows stable bonds to be formed between the heme iron and the amino acid side chains. These bonds keep hemoglobin in the Fe3+ form—a form that is unable to bind oxygen and is inaccessible to the reducing enzymes. The disorder is seen only in heterozygotes; about 30% of hemoglobin is abnormal, as detected by electrophoresis. Cyanosis is noted at birth. Hemolysis is minimal, and therapy is not needed.
The Thalassemias
The thalassemias have a worldwide distribution; in many regions, they are responsible for major medical, social, and economic perturbations. Throughout the world, the regions in which the thalassemias occur are contiguous with regions endemic for malaria, indicating that the heterozygous forms of thalassemia provide protection against malaria.104 The techniques of molecular biology have helped elucidate the patho-physiology of these syndromes,104 which in turn has enabled investigators to make unambiguous antenatal diagnoses. Using these data, expectant parents can make thoughtful, informed choices regarding the outcome of pregnancies in which the fetus is severely affected.
Molecular Genetics
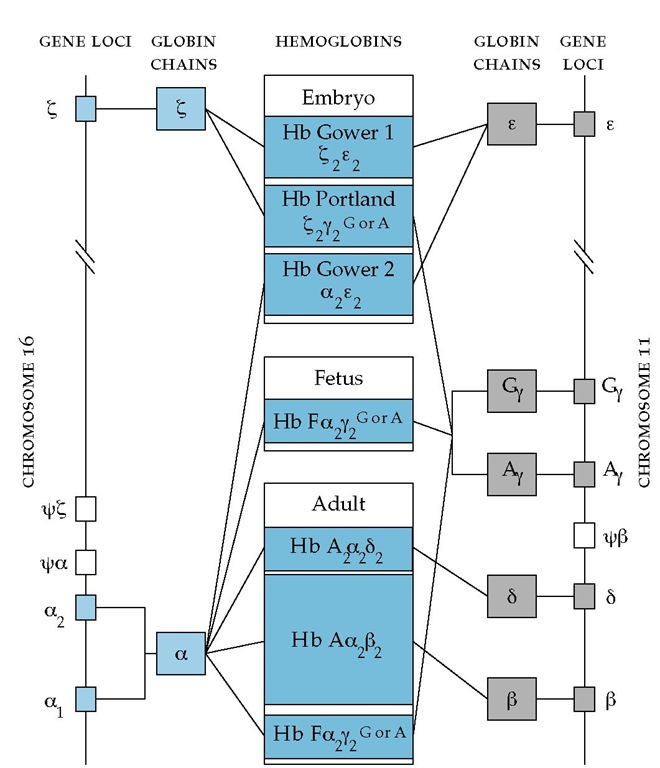
Thalassemias result from gene deletion, abnormalities in transcription and translation [see 9:VII Basic Genetics for the Clinician], and instability of the mRNA directing globin synthesis or of the globin itself. The genes controlling the synthesis of the a and non-a chains of hemoglobin are located on chromosomes 11 and 16 [see Figure 6].
Pathophysiology
In a healthy person, the synthesis of a and chains is meticulously coordinated to produce adult HbA (a2| 2). In contrast, patients with thalassemia usually demonstrate imbalanced synthesis of normal globin chains. Occasionally, however, thalassemia-like syndromes can result from diminished production of a structurally abnormal chain.105 Because one of the globin chains is present in reduced amounts, the unpaired chain accumulates in the developing erythroid precursor cell, and toxicity results [see Figure 6]. Consequently, erythroid cells die in the marrow, giving rise to a classic form of ineffective erythropoiesis [see 5:III Anemia: Production Defects]; affected erythrocytes undergo he-molysis in the peripheral blood.
The -thalassemias are characterized by diminished production of | -globin chains, causing unmatched a-globin chains to accumulate and aggregate. These aggregates of a chains precipitate, causing decreased ATP synthesis, potassium leak, and reduced amounts of surface sialic acid; the affected erythrocytes are misshapen and relatively rigid. The membrane Ca2+ barrier is breached, allowing Ca2+ to enter. These a-globin aggregates also appear to keep the K+-C1- cotransporter functioning; as a result, in severe forms of | -thalassemia, dehydration of varying degree is seen.106 The RBC membranes are unstable and fragment easily; there is evidence of oxidation of RBC membrane proteins 4.1 and spectrin [see Figure 2]; and phosphatidylserine migrates to the outer membrane layer, perhaps forming a nidus for thromboem-bolic events.107,108 These destructive alterations of the membrane, which can be detected by macrophages, may in part be caused by local oxidation.109,110 Abnormal accumulations of a chains probably account for the accelerated apoptosis and ineffective erythropoiesis seen in marrow erythroid precursors.110 The overall decrease in hemoglobin synthesis per cell accounts for the observed hypochromia and target cell formation.
Figure 6 The genes encoding the a and non-a chains that come together to form the hemoglobin tetramer lie on chromosomes 16 and 11, respectively. The a genes are present at duplicated loci. Six distinct species of normal hemoglobin have been described. Three of these hemoglobins are synthesized only during embryonic stages of development (Hb Gower 1, Hb Portland, and Hb Gower 2). HbF predominates during fetal development, and a small amount continues to be synthesized in adult life. HbA and HbA2 constitute the major forms of adult hemoglobin. Different hemoglobin genes are activated at various stages of development. In the embryo, | chains combine with e chains to yield Hb Gower 1 and with y chains to form Hb Portland; a and e chains are linked to form Hb Gower 2. There are two varieties of y chains that are derived from separate loci and that differ in a single amino acid; y contains glycine at position 136, whereas Ay contains alanine at this position. The genes coding for the two other non-a chains, | and 8, which are required for the synthesis of adult hemoglobins, are switched on late in fetal development. The factors regulating this precisely coordinated sequence of changes in hemoglobin production are poorly understood; some evidence suggests that DNA segments intervening between the various hemoglobin genes may control the relative rates of synthesis of the adjacent gene products.
Patients with a-thalassemia demonstrate accumulations of excess | chains that, if present in sufficient amounts, form | 4 tetramers (HbH) [see Figure 6]. Such tetramers have high oxygen affinity and are unstable, aggregating in the presence of oxidative stresses such as infection. | -Globin aggregates also become attached to the erythrocyte’s membrane skeleton, but they produce lesions different from those produced by a-globin aggregates. In the severe a-thalassemias, ineffective erythropoiesis is less prominent85; rather, destruction of peripheral RBCs is the critical characteristic. RBCs in severe a-thalassemia are rigid, but in contrast to those in severe | -thalassemia, the membranes in severe a-thalassemia are more stable than normal.107,108 Also in contrast to | -thalassemia, the RBCs in severe a-thalassemia are uniformly overhydrated.
Both a-thalassemia and-thalassemia are characterized by variable degrees of anemia. This variation is attributable to varying degrees of ineffective erythropoiesis and hemolysis.110 When the anemia is severe, the associated hypoxia induces a vigorous compensatory erythropoiesis, leading to expansion of the marrow cavity, osteopenia, and enlargement of reticuloen-dothelial organs; tumors may arise at sites of extramedullary erythropoietic activity. Destruction of erythroblasts and eryth-rocytes may predispose to cholelithiasis and obstructive jaundice. Patients with the more severe forms of thalassemia require regular transfusions, which may eventually generate clinically significant iron overload [see 5:II Red Blood Cell Function and Disorders of Iron Metabolism].
Diagnosis and Treatment of Thalassemia
The HbF and HbA2 measurements that aid in the diagnosis of the | -thalassemias are readily available from clinical laboratories using hemoglobin electrophoresis and, more recently, HPLC. In contrast, the tests required to diagnose the a-thalassemias are quite sophisticated and in the past were performed only in institutions specifically engaged in thalassemia research. Currently, specialized laboratories can detect the number and position of deleted a-globin genes. Further description and information on diagnostic testing is available online at http://www.geneclinics.org.
The clinical diagnostic tools used to assess patients suspected of having a-thalassemia include clinical history, smear evaluation, calculation of indices, brilliant cresyl blue staining, and family studies. In practice, a-thalassemia trait is diagnosed on the basis of a finding of microcytosis in an iron-replete patient who has normal HbA2 and HbF levels.
The diagnosis of either a- or -thalassemia should be suspected when the MCV is less than 75 fl and the RBC count is greater than 5 million cells. A patient with these two findings has an 85% chance of having a thalassemia syndrome.111 In one study, diagnosis of thalassemia was not considered in about half of the patients with the disease.
Thalassemia
Long-term transfusions eventually generate iron overload, which if untreated leads to death from cardiac hemochromatosis during adolescence. Iron overload should be managed prophy-lactically by infusion of subcutaneous deferoxamine, an iron chelator, before iron buildup occurs. Subcutaneous deferoxam-ine at a dosage of 50 mg/kg/day can effect iron losses of 50 to 200 mg/day but only if infused continuously over 8 to 12 hours for 5 days each week.113 Such therapy not only prevents left ventricular dysfunction but also reverses already established abnor-malities.114 The beneficial effects of iron chelation have improved the prognosis for persons with Cooley anemia114: it is no longer inevitable that patients die in their 20s of arrhythmia and left ventricular failure. With current deferoxamine therapy, 61% of patients born before 1976 have had no cardiovascular disease. Compliant patients whose ferritin levels are mostly below 2,500 ng/ml have a survival rate of 91% after 15 years.115 However, compliance with deferoxamine is a problem, and the cost of the drug, together with the cost of the pump and tubing that are required for administration, takes it out of reach of most patients in developing countries. Use of the oral iron chelator deferiprone remains a very contentious issue with regard to its safety and ef-ficacy.116 A new orally active tridentate iron chelator (ICL670) looked very promising in early trials, but it is currently available only on fixed protocols.117 Bone marrow transplantation has been performed with HLA-matched sibling donors. More than 1,000 patients have now undergone allogeneic bone marrow transplantation from sibling donors who either were normal or had | -thalassemia trait.118 Some patients with hemoglobin E | -tha-lassemia have a phenotype fully as severe as | -thalassemia major and require the same therapy, including allogeneic bone marrow transplantation.99 Experience with cord blood transplantation is more limited.119 Depending on the condition of the patient at the time of transplantation, the rate of transplantation-related mortality was 5% to 19%; the cure rate was 54% to 90%.120121 Other approaches to the treatment of severe | -thalassemia are still experimental.56 However, two small clinical trials have shown hydroxyurea to be of benefit. This treatment probably works by increasing the production of y chains, which combine with and remove the excess a chains, and by causing an increase in the production of HbF, a useful hemoglobin.
Thalassemia minor (thalassemia trait) Patients with thalassemia minor are usually heterozygous for a | -globin mutation and have either mild or no anemia. The peripheral smear shows distinct hypochromia and microcytosis with basophilic stippling. Splenomegaly is occasionally found.
The HbA2 level is elevated above 5% in 90% of patients, and the HbF level is raised above 2% in 50% of patients. This increase in fetal hemoglobin occurs in varying proportions per RBC (a phenomenon known as heterocellular distribution), as shown by the Kleihauer-Betke stain. Patients with higher HbF levels have less severe anemia. Heterozygotes for 6|-thalassemia produce increased amounts of HbF but only normal amounts of HbA2.
Iron deficiency anemia should be excluded from the differential diagnosis of ^-thalassemia trait [see 5:II Red Blood Cell Function and Disorders of Iron Metabolism]. Generally, it is easy to distinguish the two disorders. Both are associated with hypochromia and microcytosis, but iron deficiency produces hypoproliferation of RBCs, whereas | -thalassemia minor causes only a minimal reduction in their number. At a hemoglobin level of 9 g/dl, an iron-deficient patient has an RBC count of about 3 million cells/m1, whereas a patient with | -thalassemia trait has an RBC count of about 5 million cells/m1. If the diagnosis remains in doubt, measurement of the serum iron and iron-binding capacity or of the serum ferritin level can be used to distinguish these disorders. It is important to remember, however, that a patient with thalas-semia trait may also be iron deficient as a consequence of vaginal bleeding, gastrointestinal bleeding, or both.
Thalassemia intermedia As the term implies, thalassemia intermedia is characterized by clinical manifestations of moderate severity. Patients with this syndrome have distinct anemia, with hemoglobin levels as low as 6 to 7 g/dl; they exhibit variable degrees of hepatosplenomegaly, but they usually do not require regular transfusions. During infections or other erythro-poietic insults, however, transfusions may be needed transiently. In two small clinical trials, isobutyramide was found to be of benefit.
Thalassemia-like variants The hemoglobinopathy associated with hemoglobin Lepore represents another | -thalas-semia variant. Patients who are homozygous for this disorder present with Cooley anemia or thalassemia intermedia, and their RBCs contain only hemoglobin Lepore and HbF.
Hereditary persistence of fetal hemoglobin The RBCs of patients heterozygous for hereditary persistence of fetal hemoglobin (HPFH) contain about 50% of HbF, whereas homozy-gotes have 100% of HbF. It was once believed that patients with HPFH were well and had minimal or no anemia, but some clinical variants of HPFH associated with distinct anemia have been described.