Alteration of the erythrocyte membrane usually signals the reticuloendothelial macrophages to remove the damaged red blood cell (RBC) from the circulation. In extraordinary circumstances, however, the damage to the membrane is so great that the eryth-rocyte undergoes hemolysis, and its intracellular contents, including hemoglobin, are liberated into the plasma. This topic describes structural and functional features of normal erythro-cytes and diseases involving membrane architecture, RBC proteins, and extracorpuscular factors that can lead to shortened RBC survival.
Development, Structure, and Physiology of the Erythrocyte
Erythroid precursor cells undergo four or five cell divisions in the bone marrow and then extrude their nuclei and become reticulocytes. As these enucleate cells mature, hemoglobin synthesis decreases. The cells lose most of their transferrin receptors and enter the peripheral blood; they survive in the circulation for about 4 months.
As they move through the circulation, erythrocytes must withstand severe mechanical and metabolic stresses, deform to traverse capillaries with diameters half their own, resist high shearing forces while moving across the cardiac valves, survive repeated episodes of stasis-induced acidemia and substrate depletion, and avoid removal by the macrophages of the reticu-loendothelial system. They must also maintain an internal environment that protects hemoglobin from oxidative attack and sustain the optimum concentration of 2,3-bisphosphoglycerate (2,3-BPG) needed for hemoglobin function.
Hemoglobin
The normal adult RBC contains three forms of hemoglobin (Hb): HbA (96%), HbA2 (2% to 3%), and HbF (< 2%). Normal HbA (a2|2) is composed of two a chains, coded by four genes on chromosome 16, and two | chains, coded on chromosome 11.
HbA2 is composed of two a chains and two 6 chains (a262), and fetal hemoglobin (HbF) is composed of two a chains and two y chains (a2y2). The genes for the 6, and y chains are closely linked to one another on chromosome 11. The extraordinarily high concentration of hemoglobin in the RBC—33 to 35 g/dl (the mean corpuscular hemoglobin concentration, or MCHC)—pro-duces a viscous solution intracellularly.
Nonhemoglobin CONSOL
Erythrocytes principally utilize glucose to maintain the reducing power that protects the cell against oxidative attack, to generate the 2,3-BPG required to modulate the function of hemoglobin, and to control the salt and thus the water content of the RBC by the actions of adenosine triphosphate (ATP) and the transport adenosine triphosphatases (ATPases) [see Table 1]. The water and the hemoglobin content of the RBC determine the mean corpuscular volume (MCV) and the MCHC.
Plasma Membrane
The RBC normally has a discoid shape with a diameter of 7 to 8 M-m, an MCV of 85 to 90 femtoliters (fl) (1 fl = 10-15 L), and a surface area of 140 ^m2 [see Figure 1]. Its unique shape enables it to squeeze through capillaries as narrow as 3 ^m in diameter.
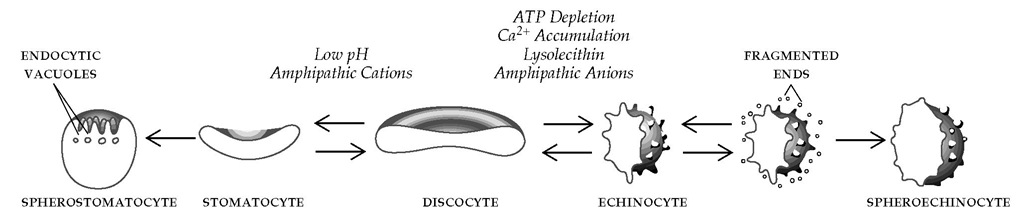
Lipids (phospholipids and cholesterol) account for 50% of the weight of the surface membrane. The phospholipids are distributed asymmetrically in the membrane bilayer, with positively charged ones in the outer half and relatively negatively charged ones predominantly in the inner half. This asymmetry permits the selective intercalation of small charged molecules into either the outer or inner half of the bilayer, producing echinocytes or stomatocytes [see Figure 1].
The RBC membrane proteins include integral and peripheral proteins. Integral proteins interact with and span the hydropho-bic phospholipid bilayer [see Figure 2]. The major integral proteins of the erythrocyte membrane are the glycophorins (which contain most of the membrane sialic acid and carry the MNSs blood group antigens) and band 3, which is the anion and bicarbonate transporter.
Table 1 Erythrocyte Metabolism
|
Pathway |
Product |
Functions of Metabolic Products |
|
Glycolysis by |
ATP |
Serves as a substrate for all kinase reactions, for the ATPase-linked sodium-potassium pump, for the ATPase-linked calcium efflux pump, and for other ATPases of the RBC membrane, including aminophospholipid translocase Maintains deformable state of RBC membrane |
|
Embden-Meyerhof pathway |
2,3-DPG |
Interacts with deoxyhemoglobin, shifting equilibrium to favor unloading of O2 from oxyhemoglobin Acts as an intracellular anion that cannot cross the RBC membrane |
|
NADH |
Acts as a substrate for a methemoglobin reductase, enabling it to reduce methemoglobin (Fe3+) to hemoglobin (Fe2+) |
|
|
Pentose phosphate pathway (hexose monophosphate shunt) |
NADPH |
Serves as a substrate for another methemoglobin reductase in methemoglobin reduction (a fail-safe mechanism) Serves as a coenzyme for glutathione reductase in reduction of oxidized glutathione; reduced glutathione (GSH) protects RBC against oxidative denaturation |
ATP—adenosine triphosphate 2,3-DPG—2,3-diphosphoglycerate NADH—reduced nicotinamide-adenine dinucleotide NADPH—reduced nicotinamide-adenine dinucleotide phosphate
Figure 1 The normal erythrocyte, or discocyte, undergoes shape changes in response to conditions created by treatment with certain agents. Most changes are reversible if inducing agents are removed before the permanent loss of membrane material.
The peripheral proteins are all found at the cytosol face of the membrane. The interaction of these peripheral proteins, which include spectrin and actin, results in the tough but resilient cy-toskeleton of the erythrocyte. The peripheral cytoskeleton, in turn, is connected to the integral proteins [see Figure 2].1,2
The membrane carbohydrates contribute to the external negative charge of the membrane and function partly as blood group antigens. Some of these glycolipids associate with phos-phatidylinositol to form a glycolipid anchor, called the glyco-sylphosphatidylinositol (GPI) anchor. These GPI anchors provide the membrane-anchoring site for several classes of proteins that have important biologic functions at membrane surfaces, including several that serve to control complement action [see Paroxysmal Nocturnal Hemoglobinuria, below].3
Control of Hydration and Volume
Control of RBC volume has considerable pathophysiologic importance because the water and cation contents of RBCs determine intracellular viscosity and the ratio of surface area to volume. The Na+ and K+ content is determined by passive diffusion and by active transport, primarily through Na+,K+-ATPase. The major in-tracellular anion is Cl-, which enters the RBC with high permeability through band 3. The K+-Cl- cotransporter drives the K+-Cl- gradient and is activated by RBC swelling and low intracellular pH, causing a net loss of K+ and Cl-. The Ca2+-ATPase actively pumps Ca2+ out of the RBC, making the free cytosolic Ca2+ content less than 0.1 ^M—four orders of magnitude lower than the plasma concentration of 1 mM. The Gardos channel, which is a Ca2+-activated K+ efflux channel, plays an important role in volume regulation. Water enters and exits through a water channel called CHIP 28 (28 kd channel-forming integral membrane protein) or aquaporin. Other important intracellular anions are 2,3-BPG and hemoglobin, neither of which penetrates the cell membrane. When the concentration of free cytosolic Ca2+ rises to levels even as low as 0.3 ^M, the channel is activated and results in a net loss of K+. If such a loss is not corrected, the affected RBC becomes dehydrated.
Shape Changes
ATP depletion, calcium ion accumulation, or treatment with lysolecithin or with anionic amphipathic compounds transforms the normal erythrocyte, or discocyte, into an echinocyte—a cre-nated spiculated cell sometimes called a burr cell [see Figure 1]. Calcium, acting either alone or in concert with the calcium-binding protein calmodulin, can effect the echinocytic shape change. If the echinocytic process persists, fragmentation or budding of the tips of the echinocyte leads to loss of membrane components, particularly of band 3 and phospholipids. This results in loss of surface area, a reduction in the ratio of surface area to volume, and the formation of poorly deformable spheroechinocytes.
Principles of Bloods Flow
The major determinants of blood flow are the hematocrit; the plasma concentration of proteins such as fibrinogen and im-munoglobulins, which influence the degree of rouleau formation or aggregation; RBC deformability; the caliber of blood vessels; and the shear rate (the ratio of flow rate to tube radius). At the low shear rates that exist in postcapillary venules, the RBCs tend to clump in asymmetrical masses, with a consequent increase in blood viscosity and resistance to flow.
Cell Aging and Death
In the bone marrow, the developing reticulocyte progressively loses its residual RNA over a 4-day period after nuclear extrusion. At the conclusion of this stage, the reticulocyte can no longer engage in protein synthesis. The active K+-Cl- co-transporter functions to reduce cell volume. With the membrane protein assembly complete, the resulting mature cell enters the circulation and survives for a period of 100 to 120 days.5 Erythrocyte death is an age-dependent phenomenon and may be related to mechanical and chemical stresses the cell encounters in the circulation. As the erythrocyte ages, it loses water and its surface area diminishes. The ratio of surface area to volume decreases and the mean corpuscular hemoglobin concentration increases, impairing cell deformability. In addition, decreased enzymatic activity lowers the cell’s ability to withstand metabolic stress. Aging may be manifested by changes at the erythrocyte’s surface, such as a decrease in the density or type of surface charge or the appearance of a senescence neoantigen, perhaps oxidatively clustered band 3 [see Figure 2], that binds specific immunoglobulins and complement compo-nents.6 By such changes, the age-worn erythrocyte signals its incapacity to the reticuloendothelial system, triggering removal by macrophages.
Under physiologic conditions, slightly less than 1% of the RBCs are destroyed each day and are replaced by a virtually identical number of new cells. For a 70 kg (154 lb) man with a blood volume of about 5 L, about 50 ml of whole blood, containing approximately 22 ml of packed erythrocytes, is destroyed and replaced each day. Inasmuch as one third of each erythro-cyte is hemoglobin, the replacement of these cells requires the synthesis of about 7 g of hemoglobin each day. Normal adult bone marrow can readily increase its erythroid output fivefold. After extensive and prolonged anemic stress, erythroid production can be raised by as much as seven or eight times. The supply of iron, however, places an important limit on RBC replacement: three fourths of the iron used in the synthesis of cells in a day comes from cells that were destroyed on the previous day.
General Features of Hemolytic Anemias
The severity of anemia is determined both by the rate of RBC destruction and by the marrow’s capacity to increase erythroid production. When a person has a healthy marrow, erythrocyte survival time can be reduced from 120 days to 20 days without inducing anemia or jaundice; however, a substantial reticulocy-tosis will be present in such cases.
Most forms of hemolysis are extravascular; the damaged cell signals its changed status to the reticuloendothelial system via its membrane and is removed. In unusual circumstances in which damage to the erythrocyte is devastating—as in some forms of complement-mediated lysis—or in circumstances in which the reticuloendothelial system cannot cope with the burden of damaged cells, intravascular lysis develops and leads to hemoglobinemia.
Hemoglobin released to the plasma is degraded to a| dimers, which bind to haptoglobin. The hemoglobin-haptoglobin complexes are removed by the reticuloendothelial system. When the haptoglobin-binding capacity is exceeded, a| dimers pass into the glomerular filtrate. Some of the a| dimers are excreted into the urine directly, producing hemoglobinuria, whereas others are taken up by renal tubule cells. Iron-containing renal tubule cells may be excreted for several days after an episode of in-travascular hemolysis. Hemosiderinuria can be identified with Prussian blue stain. Free plasma hemoglobin can dissociate into globin and hemin. Hemin may bind to hemopexin and may reach the renal tubule cells in that form, or it may bind to plasma albumin, producing methemalbuminemia.
Intravascular hemolysis may produce severe anemia acutely. In addition, erythrocytic membrane particles released into the plasma may act as potent stimuli for disseminated intravascular coagulation. Acute severe hemolysis is also a cause of acute renal failure [see 10:VI Acute Renal Failure]. When a patient compensating for a marked increase in hemolysis has an infection that sharply impairs marrow erythroid activity,7 the hemoglobin level may fall dramatically—a condition called aplastic crisis. With chronic hemolysis, pigment stones often develop in the gallbladder.
Causes of hemolysis may be classified as either extracorpuscu-lar or intracorpuscular. The intracorpuscular causes, which are essentially erythrocyte defects, comprise membrane abnormalities, metabolic disturbances, and disorders of hemoglobin structure or biosynthesis. Extracorpuscular causes represent abnormal elements within the vascular bed that attack and destroy normal erythrocytes. Because erythrocytes with intracorpuscular defects that cause hemolysis are intrinsically abnormal, when they are transfused into normal recipients, their survival time is characteristically short. Of the intracorpuscular defects, only one disorder, paroxysmal nocturnal hemoglobinuria, is not hereditary.
Erythrocyte Membrane Defects
Disorders of Salt and Water Metabolism
Hydrocytosis (Hereditary Stomatocytosis)
Hydrocytosis is a hereditary disorder that usually presents early in life as partly compensated hemolytic anemia; occasionally, the spleen is palpable. The MCV is usually elevated. The peripheral smear shows stomatocytes [see Figure 3]. Passive flux of both Na+ and K+ increases greatly. The Na+,K+-ATPase is overwhelmed; the cation concentration and thus the water content of the RBC increase, accounting for the increase in MCV and the decrease in the ratio of surface area to volume. Stomatocytes appear to adhere more avidly than normal RBCs, a finding that may account for the reported increase in thromboembolic events.8 Perhaps more importantly, the number of RBCs with phosphatidylserine exposed on the outer membrane surface is increased. Phosphatidylserine—a relatively negatively charged phospholipid that is normally found predominantly in the inner membrane layer—provides a nidus for thrombin formation and thus may also contribute to the tendency to thrombosis.9 Splenectomy may lead to improvement in the anemia. Other therapies may eventually prove useful; vaso-occlusive events were controlled in one patient by long-term RBC transfusion and in another by therapy with pentoxifylline.8
Figure 2 Band 3, the anion transport channel (orange), and the other integral proteins glycophorin A (not shown), glycophorin B (not shown), and glycophorin C (green) span the red cell membrane. Branching external carbohydrate side chains are attached to these proteins. The hydrophilic, polar heads of the phospholipid molecules that make up the bilayer are oriented toward the cell surface, whereas the hydrophobic fatty acid side chains are directed toward the interior of the bilayer. Cholesterol is intercalated between the fatty acid chains. Band 3 binds hemoglobin and glyceraldehyde-3-phosphate dehydrogenase on its cytosol surface. Spectrin (yellow), actin (red), tropomyosin (blue), and band 4.1 (light green) form a latticework on the inner membrane surface. The spectrin heterodimers associate to form heterotetramers. The lower figure depicts the hexagonal cytoskeletal lattice on the inner membrane surface. Band 2.1 (ankyrin) links the integral protein band 3 to the peripheral cytoskeleton through the | chain of spectrin. Additional linkage is provided by glycophorin C and band 4.1.
Figure 3 Stomatocytes are identified by slitlike areas of central pallor (a); the smear also shows microspherocytes, which are a more advanced stage of stomatocytosis. On scanning electron microscopy or examination of wet preparations, the microspherocytes are shown to be stomatocytes. Microspherocytes are seen in hereditary spherocytosis and in autoimmune hemolytic anemia, as well as in other conditions characterized by relatively selective loss of membrane material or increase in cell volume. Supravital stain of erythrocytes (b) shows single and multiple blue-staining Heinz bodies within counterstained erythrocytes. Phase microscopy can also be used to demonstrate Heinz bodies. Elliptocytes are visualized in a smear from a patient with hereditary elliptocytosis (c).
Xerocytosis
Xerocytosis, another hereditary hemolytic disorder, is characterized by a membrane defect that leads to loss of cations, particularly K+. Dehydration of erythrocytes occurs because the K+ leak exceeds the Na+ influx, possibly as a result of an overactive K+-Cl-cotransporter. Patients present with variably compensated he-molysis. Splenomegaly is not a prominent feature. The peripheral smear is variable, showing target cells, stomatocytes, echin-ocytes, or so-called hemoglobin puddling (i.e., hemoglobin collected around the circumference of the cell). MCHC is increased. Because these rigid cells are removed in many parts of the reticu-loendothelial system, splenectomy is of little benefit.10 In rare instances, xerocytosis can cause nonimmune hydrops fetalis.