Filariasis
Several insect-transmitted filarial nematodes cause chronic infections in humans [see Table 2]. The major filarial nematodes are responsible for lymphatic filariasis, onchocerciasis, and loiasis. Other filarial nematodes include Mansonella ozzardi, M. perstans (formerly called Tetrapetalonema perstans), and M. streptocerca (formerly called T. streptocerca); these nematode species cause fewer cases of human infection than the major filarial nematodes and are of less certain pathogenicity, but they are thought to cause bronchospastic tropical pulmonary eosinophilic syndrome.
Lymphatic Filariasis
The most common filarial infections are caused by lymphatic tissue-dwelling filarial parasites, three species of which infect humans. It is estimated that 120 million persons in 76 countries are infected with lymphatic filarial parasites and that 44 million have overt clinical disease. However, the Global Alliance to Eliminate Lymphatic Filariasis (http://www.filariasis.org) has brought the eradication of lymphatic filariasis within reach over the next decade by bringing together a strong group of collaborators whose efforts include the provision of powerful antipara-sitic drugs.
Wuchereria bancrofti, the most common lymphatic filarial parasite, is broadly distributed in tropical regions. Brugia malayi is found in areas of Southeast Asia, and B. timori is found in parts of Indonesia. Mosquitoes serve as an intermediate host for all three species of filarial nematodes and introduce infectious larvae into humans by means of their bite (the life cycle of B. malayi is illustrated in the CDC PHIL [http://phil.cdc.gov/Phil]; photograph 3379). Different mosquito species are responsible for transmission in different areas; the major vectors include Culex, Anopheles, Aedes, and Mansonia species. The larvae develop into adult worms, which reside within lymphatic vessels and tissues. Offspring of the adult worms, called microfilariae, are covered by sheaths and may circulate in the bloodstream. In most endemic regions, microfilariae of W. bancrofti circulate in the bloodstream in greatest numbers during the night; in the South Pacific, however, no pronounced diurnal variation occurs. The time of day when the greatest numbers of microfilariae circulate correlates with the time of day when the principal mosquito vectors feed. Thousands of mosquito bites are probably required to produce an infection that results in patent microfilaremia. The incubation period until patent microfilaremia for W. bancrofti develops ranges from 3 months to the more usual 8 to 12 months.
Pathogenesis and clinical features Most clinical manifestations of lymphatic filariasis are attributable to inflammatory reactions caused by the adult worm; the immunopathogenesis, however, is poorly understood.50 Many patients with microfilaremia experience no symptoms, although lymphadenopathy of the axillary, epitrochlear, and inguinal-femoral areas is common.
The more prominent clinical manifestations of lymphatic fila-riasis may be divided into two categories: inflammatory and obstructive. Inflammatory manifestations, which often become recurrent, include lymphadenitis, lymphangitis, funiculitis, orchi-tis, and epididymitis.50 A typical presentation is the triad of lymphadenitis, lymphangitis, and fever. In contrast to bacterial ascending lymphangitis, the lymphangitis of filariasis characteristically progresses centripetally down involved extremities. Staphylococcal and streptococcal superinfections are commonly the cause. Fever is variable and may be associated with frank rigors; the patient’s temperature may reach 40° C (104° F). Systemic symptoms, such as nausea and vomiting, develop in many of these recurrent episodes. Urticaria and areas of cutaneous erythema commonly develop. The episodes may continue for as long as 7 to 10 days before spontaneously resolving. In filariasis caused by Brugia species, suppurative abscesses may form in areas of involved lymphatic channels.
The obstructive phase of filariasis usually develops after decades of exposure to the parasite and reflects lymphatic compromise, the mechanism of which is poorly understood. Inflammatory features may coexist with the obstructive phase. Features of the obstructive phase include hydrocele formation, chyluria, elephantiasis of the extremities (shown in the CDC PHIL [http://phil.cdc.gov/Phil]; photograph 373), and, less commonly, elephantiasis of the breast.50
Tropical pulmonary eosinophilia develops in some individuals infected with lymphatic tissue-dwelling filarial parasites.51 This illness is characterized by paroxysmal asthmalike attacks that are often nocturnal; by blood and pulmonary eosinophilia;and by elevated titers of antifilarial IgG, IgM, and especially IgE. Tropical pulmonary eosinophilia should be distinguished from the transient eosinophilic pneumonitis termed Loffler syndrome, which results from a transpulmonary migration of larval forms of Ascaris and, less commonly, hookworm and Strongyloides [see Intestinal Nematode Infections, above].
Laboratory findings Definitive diagnosis of lymphatic fila-riasis requires the demonstration of microfilariae or microfilarial antigens in the blood.52 Stained blood smears may reveal microfi-lariae, but often, larger volumes of blood need to be examined either by centrifugal concentration after erythrocyte lysis or by filtration through 3 ^m polycarbonate (Nuclepore) filters. In endemic areas where W. bancrofti microfilariae display nocturnal periodicity, detection of microfilariae requires examination of blood obtained after midnight or 60 to 90 minutes after daytime administration of a 2 mg/kg provocative dose of diethylcar-bamazine (care should be taken if the patient is from a region where onchocerciasis is endemic, because diethylcarbamazine may precipitate a severe reaction as a result of lysis of Onchocerca microfilariae). Because microfilarial antigen of W. bancrofti can be found in the blood throughout the day, antigen measurement is more convenient than direct microfilaria determination. Microfi-lariae are rarely found in patients with elephantiasis or with tropical pulmonary eosinophilia and often cannot be found in patients with inflammatory or obstructive filarial manifestations. In males, ultrasonography can detect adult filariae within scrotal lymphatics.50 Serologic tests may be helpful in making a diagnosis. Mild eosinophilia and elevations of serum IgE are common in lymphatic filariasis.
Treatment Lymphatic filariasis can be treated with diethyl-carbamazine (2 mg/kg orally three times daily for 2 to 3 weeks). Treatment with ivermectin or a single dose of diethylcarbam-azine (6 mg/kg)50 is also effective at clearing microfilaremia, and the combination of these two anthelmintics has led to rapid and long-term clearance of microfilariae. There is insufficient reliable research to ascertain whether albendazole, alone or in combination with diethylcarbamazine, is an effective treatment for lymphatic filariasis,53 but there is growing evidence that such treatment helps to suppress microfilaremia in patients from endemic areas. These short-term and combination therapies have been used more often in treatment of populations in filariasis control projects; for individual treatment, most experts favor the use of diethylcarbamazine for 2 to 3 weeks. Diethylcarbamazine must be used cautiously in patients exposed to Loa loa or Onchocerca volvulus, because patients infected with these filariae tend to have severe reactions to this drug.
Onchocerciasis
O. volvulus, the causative agent of onchocerciasis (river blindness), is found in equatorial Africa and in elevated regions of Mexico and Guatemala, with smaller foci in Yemen, Brazil, Ecuador, and Venezuela. It is estimated that 17 million people are infected. Simulium species (blackflies) transmit the infection. Onchocerciasis control programs, which are based on community therapy with ivermectin, have led to a substantial reduction in the incidence of disease. Repeated therapy is necessary, however, and control programs will have to continue for decades to eliminate onchocerciasis altogether.
Onchocerciasis typically occurs within several kilometers of rapidly flowing rivers and streams where blackflies breed. The life cycle of O. volvulus is similar to that of species causing lymphatic filariasis [see Figure 10]. Adult worms, however, reside in subcutaneous tissues, often enclosed in fibrous nodules. Microfi-lariae, which in this species lack an enveloping sheath, are released from female adults and localize in skin and subcutaneous tissues. Symbiotic endobacteria (of the genus Wolbachia) in filaria may cause some of the inflammatory damage of onchocerciasis.
Figure 10 Life cycle of Onchocerca volvulus, which causes river blindness and severe skin disease.
Clinical features The skin is frequently involved in on-chocerciasis, and pruritus is the most common clinical manifestation. With time, such complications as wrinkling, loss of elastic tissue, hypopigmentation or hyperpigmentation, papulovesicu-lar lesions, and localized areas of eczematoid dermatitis may develop. Firm, nontender nodules containing adult worms surrounded by fibrous tissue are often palpable in subcutaneous tissues. In Central America, nodules commonly occur on the head; in Africa, nodules are more common over bony prominences of the body. Regional lymphadenopathy also develops.
Ocular involvement is characteristic of onchocerciasis and may result in blindness. Conjunctivitis with photophobia is common. Punctate keratitis, which is caused by the accumulation of inflammatory cells around dying microfilariae, may develop within the cornea and usually resolves without consequence. However, sclerosing keratitis and chorioretinal lesions may ensue and are the major causes of onchocercal blindness. Anterior uveitis, iridocyclitis, and, less frequently, optic nerve lesions may develop as well.
Laboratory findings The principal method of diagnosis involves finding microfilariae in the skin. A small piece of superficial skin obtained by excision or punch biopsy is weighed and then incubated for several hours in saline or tissue culture media. Microfilariae that exit the skin sample are then counted in the fluid. Care should be taken not to contaminate the skin with blood that might harbor microfilariae of other species. Skin snip sites can include scapular and gluteal areas and, in Africa, leg areas. A count of more than 100 microfilariae/mg of skin indicates a heavy infection.
Another diagnostic method involves administering a 50 mg provocative dose of diethylcarbamazine; the subsequent onset of symptoms, which may include pruritus, rash, fever, and conjunctivitis, constitutes the Mazzotti reaction, which strongly suggests a diagnosis of onchocerciasis. Caution must be exercised in the use of this test because heavily infected patients may experience serious adverse reactions.
Eosinophilia is often prominent during onchocerciasis. Serol-ogy can be helpful when parasite demonstration is difficult.
Treatment Therapy for onchocerciasis improved dramatically with the introduction of ivermectin. Single-dose ivermectin therapy is free of most of the immediate cutaneous, ocular, and systemic reactions that complicated therapy with diethylcarbam-azine, the agent previously used to treat onchocerciasis. Iver-mectin, given orally in a single dose of 150 ^g/kg, leads to symptomatic improvement and clearance of microfilariae from the skin. This dose is repeated every 3 to 12 months for 3 to 4 years.19 Albendazole combined with ivermectin only transiently halts production of microfilaria, but doxycycline (200 mg daily for 4 to 6 weeks) interrupts microfilaria production for 18 to 24 months.54 Doxycycline may act by depleting adult filaria of Wolbachia en-dosymbiont bacteria, which correlates with an interruption of embryogenesis.
Loiasis
Loiasis, which is caused by the filarial nematode Loa loa, occurs in the rainforest areas of central and western Africa and is transmitted by Chrysops species of horseflies and deerflies. Adult worms reside in subcutaneous tissues (the life cycle of Loa loa is illustrated in the CDC PHIL [http://phil.cdc.gov/Phil]; photograph 3399). The microfilariae, which are sheathed, circulate in the bloodstream with a diurnal periodicity in which peak levels are reached at about noon.
Many residents of endemic areas who have loiasis have asymptomatic microfilaremia. The prominent clinical presentations are related to migrations of adult worms. In the subcutaneous tissues, migrations may produce recurrent lesions termed Calabar swellings, which are erythematous areas of swelling and edema up to 10 cm in diameter that resolve after 1 to 3 days. Infection may also present dramatically when the worm migrates subconjunctivally across the eye.
In contrast, patients who acquire loiasis after brief stays in central and West Africa exhibit different clinical and immuno-logic responses.55,56 They are usually free of detectable microfi-laremia but may experience more severe and pruritic episodes of angioedema and have brisk immunologic responses, including elevated antifilarial antibody titers, an elevated serum IgE level, and prominent eosinophilia—often greater than 3,000 eosinophils/ m1.
Parasitologic diagnosis of loiasis is made by demonstrating adult worms in subconjunctival or subcutaneous tissues or demonstrating microfilariae in the blood by means of a Nucle-pore filter concentration technique. In contrast to Wucheria mi-crofilaremia, Loa loa microfilaremia is found throughout the day. Microfilaremia may not be detectable, however. Eosinophilia may be marked, with elevations of 50% or greater. Filarial antibody titers are usually elevated. In the absence of detectable mi-crofilaremia, the diagnosis is suggested by clinical features, a history of exposure, and eosinophilia.
Therapy consists of diethylcarbamazine (6 mg/kg/day) orally for 3 weeks and may require adjunctive antihistamines or cor-ticosteroids.19 Patients with microfilaremia should be treated initially with escalating doses of diethylcarbamazine for 3 days (one 50 mg dose on the first day, three 50 mg doses on the second day, and three 100 mg doses on the third day), followed by the full 3-week course. Albendazole therapy is useful to reduce microfilaremia in patients who cannot tolerate diethylcarbam-azine. Some patients require repeated courses of therapy.57
Side effects are usually mild and include frequent occurrences of pruritus and the development of subcutaneous nodules soon after initiation of therapy. Patients with severe microfilaremia (> 50,000 microfilariae/ml) have developed fatal encephalopa-thy after diethylcarbamazine or ivermectin therapy. Diethylcar-bamazine, given in a dosage of 300 mg orally once a week, is an effective chemoprophylactic agent against loiasis for persons who are planning long-term visits to areas of Africa where this infection is endemic.
Zoonotic Filarial Infections
Although no human filarial parasites are indigenous to the continental United States, a variety of filarial parasites infect animals. In rare cases, these organisms may be transmitted via insect vectors to humans. Within the human host, they may develop into adult worms, which localize in the same organs in humans as in the definitive animal hosts. In this way, dog heartworm (Dirofilaria immitis), endemic in dogs along the Atlantic and Gulf coasts, in the Mississippi Valley, and in California, localizes to the human pulmonary arteries. A granuloma-tous response develops around the worm, producing a pulmonary nodule. Some patients experience chest discomfort, malaise, low-grade fever, cough, and, occasionally, hemoptysis. Typically, however, the pulmonary lesions are detected as a coin lesion on a chest x-ray.59 Prominent blood eosinophilia is absent, and serologic tests for filariasis are negative. In the absence of reliable diagnostic tests for human pulmonary dirofilariasis, exci-sional biopsy serves both diagnostic and therapeutic purposes.
Other zoonotic filarial parasites include D. repens, which causes subcutaneous abscesses, and B. beaveri, which produces focal lymphadenopathy. No chemotherapy is required for these infections or for infections caused by D. immitis. Generalized lymph-edema has developed in one immunodeficient child with a zoonotic Brugia infection.60
Trematode Infections
Schistosomiasis
Schistosomiasis, a chronic trematode (fluke) infection of humans, constitutes a major worldwide health problem: 200 million persons are infected, 120 million are symptomatic, and 10 million have severe disease.61 Three major species—Schistosoma mansoni, S. japonicum, and S. haematobium—infect humans. S. mansoni is found in Africa, the Arabian Peninsula, South America, and parts of the Caribbean; S. japonicum is found in Japan, China, and the Philippines; and S. haematobium is found in Africa and the Middle East. Two minor species, S. mekongi and S. inter-calatum, are found in mainland Indochina and central West Africa, respectively. Transmission of schistosomiasis cannot occur in the United States because of the absence of the specific freshwater snail that is a requisite intermediary host [see Figure 11]. However, the disease may be encountered in immigrants or travelers from endemic areas.62,63
Diagnosis
The diagnosis of schistosomiasis is suggested by a history of possible exposure, even exposure that occurred many years ago, along with compatible gastrointestinal or urinary tract symptoms, hepatosplenomegaly, eosinophilia, or a combination of these findings.
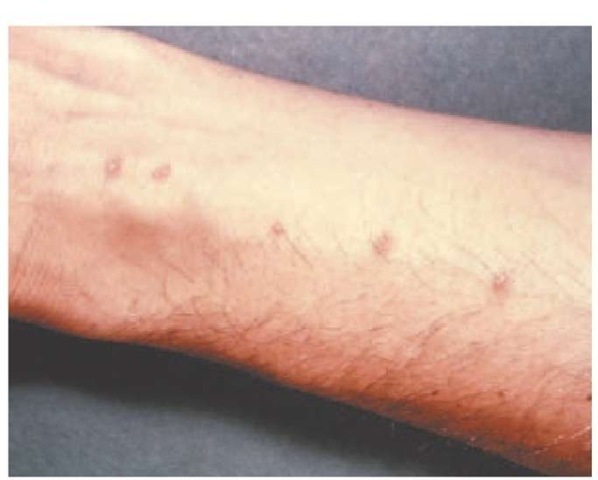
Clinical features Three stages of disease may occur in schis-tosomiasis. The first stage, schistosomal dermatitis, may develop acutely within a day of cercarial penetration of the skin. Because this entity develops early after exposure, it usually will have subsided in patients before they are seen by physicians in the continental United States. Swimmer’s itch, a similar reaction caused by exposure to animal schistosomes in freshwater and saltwater, is seen in the United States [see Figure 12].64 The schistosomes penetrate human skin and then die, causing no further infection.
Figure 11 Freshwater snails and the human body provide the living environment of the schistosomes Schistosoma mansoni, S. japonicum, S. haematobium, S. mekongi, and S. intercalatum. Eggs of each species hatch into schistosomal miracidia, which enter snails. Free-swimming cercariae released by the snails rapidly penetrate human skin and become tailless schistosomula, which enter the blood vessels. The schisto-somula mature in the intrahepatic portal blood, form male-female pairs, and begin to mate. The pairs, still copulating, migrate to various sites: S. mansoni, S. intercalatum, and S. mekongi ultimately lodge in the blood vessels of the lower intestine; S. japonicum lodges in blood vessels throughout the intestine; and S. haematobium settles in blood vessels around the bladder. Adult worms subsequently begin laying eggs in the wall of the urinary bladder or intestine. From the intestinal sites, the eggs may be transported in the portal circulation to the liver, or they may be excreted in the feces. S. hematobium, by contrast, is excreted in the urine and does not tend to lead to liver disease but rather causes local bladder damage.
The second stage of disease, acute schistosomiasis, or Kataya-ma fever, develops 4 to 8 weeks after heavy (presumably, primary) infection. This stage is thought to be caused by a severe allergic response at the onset of egg-laying by the schistosomes. Patients have fever, cough (up to 10% also have pulmonary nodules), hepatosplenomegaly, malaise, myalgias, urticaria, and eosinophilia.65-67 Deaths have ensued. Katayama fever is more severe in infection with S. japonicum than with other species, because of the high quantities of eggs produced by S. japonicum.
Chronic schistosomiasis, the third stage, is caused by the heavy deposition of eggs in the intestine or bladder and in the liver. In S. haematobium infection, the principal symptoms are hematuria, dysuria, and frequent urination. Hydronephrosis and pyelonephritis may develop as a result of fibrosis and infection. In S. mansoni, S. mekongi, or S. japonicum infection, manifestations may include fever, malaise, abdominal pain, diarrhea, blood in stools, and hepatosplenomegaly. Presinusoidal hepatic trapping of S. mansoni, S. mekongi, or S. japonicum eggs and the consequent granulomatous reaction induce portal hypertension and collateral esophageal varices. Eggs may then be shunted from the liver to the lung, with the possible sequela of pulmonary hypertension. Death may occur as a result of variceal bleeding. Hepatic encephalopathy rarely develops because the hepatic parenchyma is spared. Coinfection with S. mansoni and hepatitis B or C virus is associated with accelerated clinical dete-rioration.61 Less common sequelae of chronic schistosomiasis include intestinal polyps, bladder carcinoma, and persistent Salmonella infections. An uncommon sequela of both acute and chronic schistosomiasis is focal neurologic dysfunction from aberrant localization of eggs in CNS tissue. Embolic deposition of S. japon-icum eggs may produce cerebral granulomas, whereas S. haema-tobium and S. mansoni eggs may cause transverse myelitis involving the midthoracic or lumbar spinal cord.68,69
Laboratory findings and imaging studies Computed tomography, magnetic resonance imaging, or ultrasonography may detect hepatic periportal fibrosis and calcification, colonic wall calcifications, and changes in the bladder and ureter resulting from schistosomiasis. Serologic tests can help confirm the diagnosis; an indirect immunofluorescent test for gut-associated schistosome antigens is especially sensitive for the detection of acute schistosomiasis. Stool examination should include a search for eggs of all Schistosoma species [see Figure 13]. Urine specimens for detection of S. haematobium should be obtained between 10 A.M. and 2 P.M. If stool and urine specimens are negative, microscopic examination of biopsy specimens of rectal or bladder mucosa may demonstrate eggs of all species. Detection of circulating antigens from adult worms and eggs are promising techniques that may supersede traditional egg demonstration.
Figure 12 Swimmer’s itch is a cutaneous reaction caused by exposure to animal schistosomes in freshwater and saltwater. The schistosomes penetrate the skin and then die, causing no further infection.
Treatment
Praziquantel is used to treat infection caused by any of the five Schistosoma species; it reliably cures 60% to 90% of infected persons and reduces egg burden substantially in most others. For S. haematobium and S. mansoni, two oral doses of 20 mg/kg are given in 1 day. For S. japonicum and S. mekongi, the dosage is 20 mg/kg given orally three times in 1 day. The efficacy, the paucity of side effects, and the convenience of single-day therapy make praziquantel the drug of choice for all forms of schisto-somiasis.19 Resistance to praziquantel has been reported in S. hematobium and S. mansoni infections in Egypt and Kenya but has not yet become a widespread problem.61 In addition to pra-ziquantel, corticosteroids are beneficial for patients with spinal cord schistosomiasis59 and acute schistosomiasis.