General Management
Therapeutic principles
Four principles govern the treatment of glomerular disease. First, treat any acute complications of renal failure such as pericarditis; hyperkalemia; acidosis; hypertension with or without myocardial, cerebral, or other vascular bed crisis; and volume overload causing pulmonary edema. Second, use disease-specific therapeutic agents to treat any underlying primary and secondary glomerular disease. Third, to reduce the risk of further glomerular damage, use nonspecific strategies to lower the intra-glomerular capillary pressure, reduce systemic blood pressure, and decrease elevated serum cholesterol levels. Fourth, anticipate and treat potential complications of nephrotic syndrome, such as hypercoagulability, infection, and skeletal abnormalities.
Nonspecific treatment
To reduce glomerular filtration pressure and proteinuria, give angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor blockers (ARBs), or both. The treatment goal is to reduce proteinuria to less than 1 g/24 hr. A 24-hour urine collection remains the gold standard for assessing proteinuria, but for patient convenience, measurement of the protein-to-creatinine ratio in a spot urine specimen can be used to follow proteinuria. In such cases, both protein and creatinine concentration are measured in mg/dl. Dividing the protein concentration by the creati-nine concentration yields an estimate of the amount of protein excreted in grams per 24 hours.
Hypertension requires aggressive treatment. The goal is a blood pressure of less than 130/80 mm Hg. Hyperlipidemia is treated with a statin drug. The goal is a low-density lipoprotein (LDL) cholesterol level of less than 100 mg/dl. In addition to lowering lipid levels, statins have been shown to reduce protein-uria and to help prevent progression of glomerular disease by lipid-independent mechanisms.
Specific Glomerular Diseases
Diseases presenting as nephritic syndrome
The clinical features of nephritic syndrome are caused by a severe and typically acute inflammatory injury to the glomeruli, which leads to decreased renal function. The clinical characteristics include hematuria, proteinuria, hypertension, and increased creatinine levels. Hematuria in nephritic syndrome may be macroscopic or microscopic.15 RBCs and RBC casts in the urine, which represent an active urinary sediment, are pathognomonic for glomerulonephritis. Proteinuria in nephritic syndrome is generally less than 3.5 g/24 hr. In contrast, nephrotic syndrome is distinguished by a marked increase in glomerular permeability and more marked proteinuria (> 3.5 g/24 hr).
The major complications of nephritic syndrome are hypertension, volume overload, hyperkalemia, and metabolic acidosis; patients may present with one or more of these. The decrease in glomerular filtration in nephritic syndrome causes marked salt and water retention, leading to hypertension and edema. Acute hypertensive crisis with congestive heart failure, cerebral failure, and pulmonary edema may occur. An acute decrease in GFR can also cause hyperkalemia and metabolic acidosis.
The more common forms of glomerulonephritis that present as nephritic syndrome can be classified on the basis of their underlying disease mechanisms into three categories: immune complex mediated (e.g., postinfectious glomerulonephritis, IgA nephropathy), antibody induced (e.g., anti-GBM disease), and pauci-immune (i.e., with no immune complexes detected by immunofluorescence; for example., vasculitis associated with anti-neutrophil cytoplasmic antibodies [ANCAs]). The clinical course of individual glomerular diseases may vary. For example, PSGN is usually acute and self-limiting, IgA nephropathy is intermittent and chronic, and anti-GBM disease and ANCA-associated vasculitis have a rapid onset and are progressive, leading to end-stage renal failure within a short period if not treated.
Glomerular Diseases Associated with Infection
Acute glomerulonephritis can result from bacterial infections (Streptococcus, Staphylococcus aureus, Salmonella, Escherichia coli, Legionella, and Mycoplasma), viral infections (hepatitis B, hepatitis C, HIV, and cytomegalovirus), parasitic infections (Schistosoma, Plasmodium, Trypanosoma), and fungal infections (Aspergillus, Histoplasma, Candida). Renal disease associated with infection is typically immune complex related; the complexes comprise antigens from the infectious agent and host antibodies directed against the antigens.
The classic glomerular disease associated with infection is PSGN, which produces a different glomerular histologic picture than most other postinfectious forms of glomerulonephritis.16 At present, hepatitis C17 and HIV18,19 are the leading causes of infection-associated glomerulonephritis in developed countries, whereas PSGN remains common in other parts of the world. Hepatitis C-associated glomerulonephritis and PSGN typically present as nephritic syndrome, whereas HIV causes nephrotic syndrome.
Hepatitis C virus-associated glomerulonephritis Glomerular disease is a common extrahepatic manifestation of chronic hepatitis C infection. Hepatitis C infection is now the major cause of essential mixed cryoglobulinemia. Patients can present with different histologic and clinical syndromes,20 with renal disease carrying the worst prognosis. About 40% to 50% of patients with renal disease have systemic indications of cryoglobuline-mia, manifesting as the triad of arthralgias, purpura, and weakness; 50% of patients have renal manifestations only, in the form of proteinuria, microhematuria, or renal dysfunction. The pro-teinuria may be nephrotic (> 3.5 g /24 hr) or nonnephrotic (< 3.5 g/24 hr). The majority of patients have hypocomplementemia (low C3 and C4 levels) and elevated rheumatoid factor levels (indicative of cryoglobulins). Occasionally, patients present with signs of severe vasculitis and a rapid decline in renal function. Overt liver disease is unusual, however.
The most common histologic finding in patients with hepatitis C-associated glomerulonephritis is MPGN type I with mixed cryglobulinemia, but there are also reports of MPGN type I without cryoglobulinemia in these patients [see Membranoprolifera-tive Glomerulonephritis, below]. MPGN is characterized by deposits of preformed hepatitis C antigen-antibody complexes that are trapped in the glomerular subendothelial space and mesan-gial cells, which causes glomerular cell proliferation. The mesangium increases in size and interposes into the GBM, causing basement membrane splitting (hence the term membra-noproliferative). Less frequently, hepatitis C causes membranous nephropathy, fibrillary glomerulonephritis, FSGS (in African Americans) and thrombotic microangiopathy (in renal transplant recipients).
Treatment of hepatitis C-associated MPGN is directed against the virus and consists of polyethylene glycol (PEG) interferon al-pha-2b and ribavirin (if renal function allows) to reduce the viral burden. Severe acute disease is treated with plasmapheresis to remove cryoglobulins, plus immunosuppressive therapy with corticosteroids and cyclophosphamide, followed by initiation of antiviral therapy.
Poststreptococcal glomerulonephritis PSGN is typically a self-limiting disease that develops after a pharyngeal infection or skin infection (e.g., impetigo or pyoderma) with so-called nephri-togenic strains of group A streptococci. Occasionally, cases have been associated with group C streptococci. PSGN most often affects children 2 to 10 years of age and is twice as common in males as in females. The disease is usually sporadic but can occur in epidemics.
PSGN is a classic immune complex-mediated form of glomerulonephritis.21 The glomerular injury is induced when circulating preformed immune complexes (comprising the strepto-coccal antigen and an antibody directed against it) are deposited in the mesangium and subendothelium. Two streptococcal antigens are most likely involved in the pathogenesis of PSGN: plas-min receptor protein (related to preabsorbing antigen and en-dostreptosin) and streptococcal zymogen (exotoxin B). Antibodies to these antigens correlate with clinical disease; both antigens have been localized in glomeruli in a large percentage of patients. The formation of immune complexes explains the typical 2- to 3-week delay between streptococcal infection and the onset of PSGN.
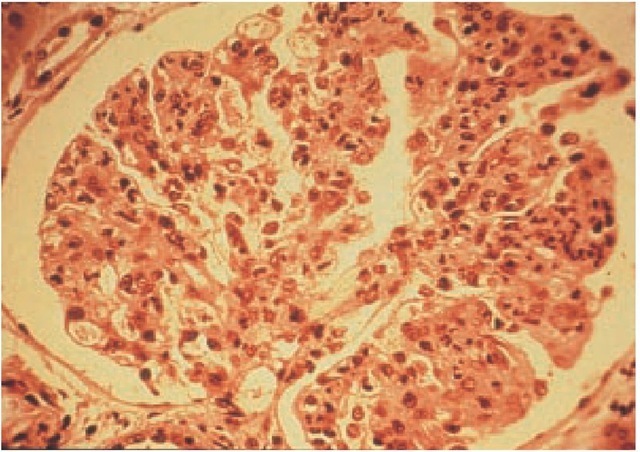
On light microscopy, PSGN is characterized by diffuse glomerular involvement [see Figure 2]. The histologic hallmark of PSGN is glomerular hypercellularity, which is caused by the proliferation of resident mesangial and endothelial cells and an influx of infiltrating cells such as neutrophils. Severe PSGN, which occurs in less than 5% of patients, is marked by crescent formation in the Bowman space. The clinical presentation of patients with crescents on renal biopsy is typically that of RPGN (see below).
Figure 2 Light micrograph of poststreptococcal glomerulonephritis showing increased glomerular hypercellularity.
There are three patterns of immunofluorescent staining in PSGN; these are termed starry sky, garland, and mesangial. The classic starry-sky pattern of immune deposition is seen in about 30% of PSGN cases and occurs early in the disease. It consists of fine granular deposits of IgG and C3, without C1q and C4, on all capillary walls and the mesangium. The garland pattern, which consists of coarse granular deposits of large amounts of IgG and C3 along the capillary loops, with relatively few mesangial deposits, also is found in about 30% of patients. The mesangial pattern of IgG deposition, which is often associated with mesangial cell proliferation, occurs in about 40% of patients.
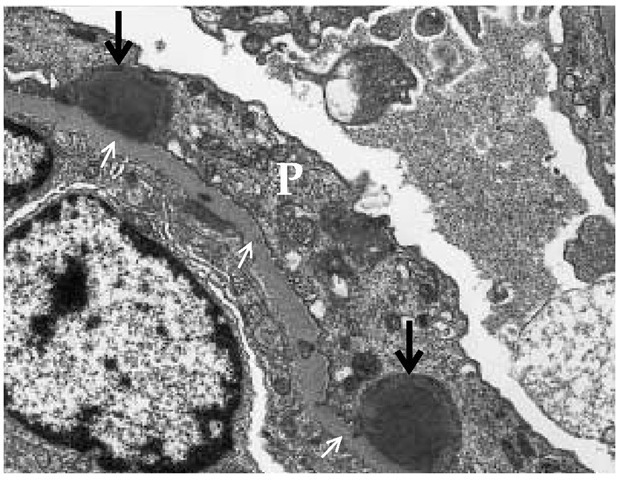
Electron microscopy in PSGN shows multiple large, dome-shaped, immune complex deposits that are referred to as subep-ithelial humps because of their location at the base of the podocyte [see Figure 3].22 The differential diagnosis of subepithe-lial immune deposits includes membranous nephropathy, lupus nephritis, and MPGN type III [see Membranoproliferative Glomerulonephritis, below]. Biopsies taken early in the course of PSGN may show immune complex deposits in the mesangium and subendothelium.
Although PSGN presents as an acute disease, there is a latent phase between the initiating pharyngitis (mean, 10 days) or skin infection (mean, 21 days) and the onset of the nephritic syndrome.16 Patients present with pronounced edema, often of the upper body; diminished urine output; dark or smoky urine containing RBCs and RBC casts; and signs and symptoms of hypertension.
The serum creatinine level is often normal; a rise in creatinine reflects underlying acute renal failure. Proteinuria is typically less than 500 mg/day during the acute illness. Throat and skin cultures are usually negative, but serologic measures of recent streptococcal infection, such as antistreptolysin O, are elevated and remain so for 3 to 6 months. Antistreptolysin O titers may be blunted by recent antibiotic usage and are less marked after skin infections. Because PSGN is induced by immune complexes via the alternative pathway, patients demonstrate marked decreases in levels of CH50 and C3, but not of C1q, C2, or C4, which are activated by the classical complement pathway [see 6:II Innate Immunity]. Depression of the C3 level that lasts longer than 8 weeks should suggest another cause.
Figure 3 Transmission electron micrograph (magnification: x 10,500) showing characteristic subepithelial hump-shaped deposits (illustrated by thick arrows) in postinfectious glomerulonephritis lying below the podocytes (P); small arrows indicate the glomerular basement membrane.
There is no role for antibiotic therapy in PSGN, because the inciting infection has typically occurred weeks earlier and the antigen-antibody complexes have already formed. Treatment is therefore supportive. The majority (> 95%) of patients recover normal renal function; the remainder, however, develop end-stage renal failure. Although macroscopic hematuria and edema usually resolve within 2 weeks, microscopic hematuria may persist for a year; 20% of patients develop nephrotic-range proteinuria, typically during the recovery phase of the disease, that resolves spontaneously. However, proteinuria in the non-nephrotic range can last as long as 2 years. Recurrent PSGN is exceedingly rare.
Other forms of postinfectious glomerulonephritis Bacterial infections (e.g., infective endocarditis,23 infected shunts,24 and visceral abscesses25), as well as certain viral infections (e.g., hepatitis B and hepatitis C26), can cause MPGN, which is associated with alternative-pathway hypocomplementemia characterized by low C3 and normal C4 levels. Antibiotic therapy eliminates infection in endocarditis and allows for resolution of the immune response. A persistently low C3 level suggests that infection is not cleared.
IgA Nephropathy
IgA nephropathy derives its name from the glomerular deposition of IgA, which causes an injurious inflammatory response.27 IgA nephropathy is the most prevalent pattern of glomeru-lonephritis found in countries where renal biopsy is widely used as an investigative tool.28 IgA nephropathy is particularly prevalent in Asia and southern Europe but is less common in the United States and Canada, which suggests a possible genetic predisposition to the disease. IgA nephropathy affects more males than females (3-to-1 ratio) and occurs most commonly in children and young adults. Although most cases of IgA nephropathy are idiopathic, a number of diseases have been associated with secondary IgA nephropathy [see Table 2], and hereditary forms have also been described.
Pathogenesis and histology Mesangial cell injury in IgA nephropathy results from the deposition of IgA on mesangial cells [see Figure 4].29 The tendency for flares of macroscopic or microscopic hematuria to occur within 24 hours after a mucosal infection (e.g., upper respiratory tract or bowel) indicates that the glomerular disease may be initiated by an impaired mucosal IgA response to infection. However, studies have suggested that IgA derived from bone marrow may be increased in these patients and that hepatic clearance of IgA may be decreased, which may in part reflect abnormal glycosylation.
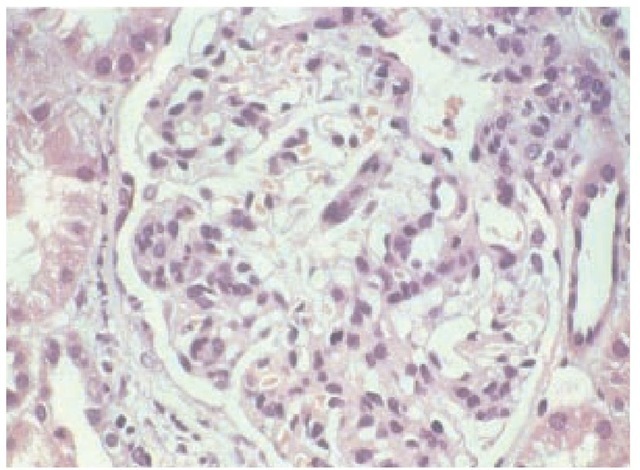
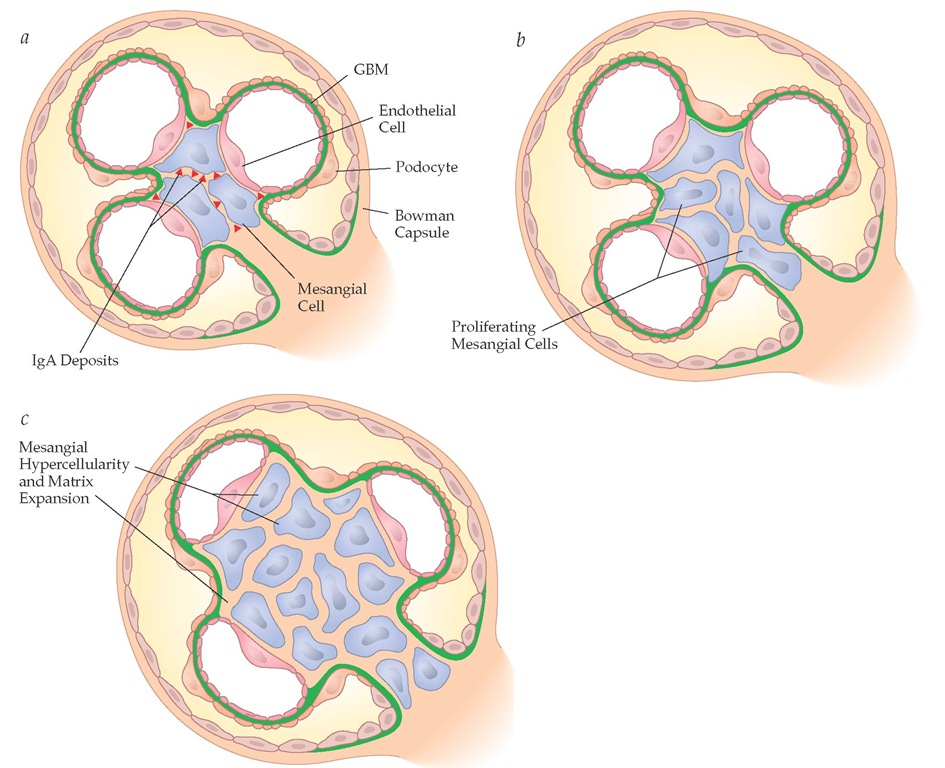
The glomerular lesions that follow mesangial cell IgA deposition are variable.29 The characteristic lesion is mesangial cell proliferation [see Figure 5], which appears on light microscopy as mesangial cell hypercellularity [see Figure 6]. Glomerular involvement may be focal or diffuse. In severe cases that progress to end-stage renal failure, mesangial scarring is noted. Glomeru-lar crescents form occasionally, and as in most forms of glomeru-lar disease, tubulointerstitial fibrosis is present in those patients with renal failure.
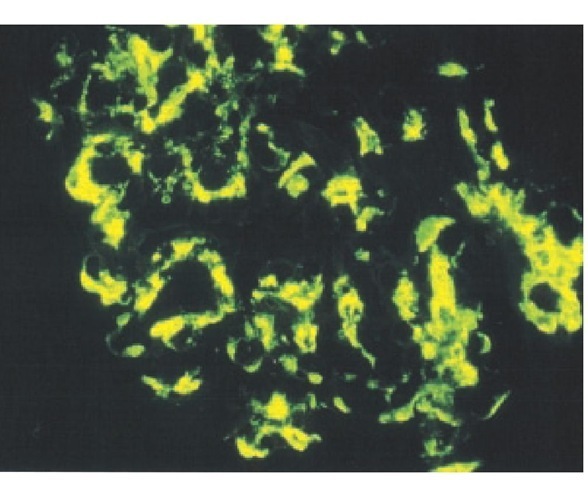
Immunofluorescent staining reveals IgA [see Figure 4] and, occasionally, IgG and IgM in the mesangium; C3 is often detected without C1q and C4, suggesting activation of the alternative complement pathway. Mesangial immune deposits can occasionally be seen as electron densities on electron microscopy.
Clinical and laboratory findings IgA nephropathy has six different clinical-presentation patterns31: episodic macroscopic hematuria, asymptomatic microscopic hematuria and non-nephrotic-range proteinuria, nephrotic syndrome, acute renal failure, chronic renal failure and hypertension, and concomitant systemic disease.
Episodic macroscopic hematuria is the classic presentation of IgA nephropathy. It occurs in 40% to 50% of patients. The hema-turia is often characterized by RBC casts and occurs within 24 hours after a mucosal infection, commonly of the upper respiratory tract. This presentation is also called synpharyngitic hema-turia. Episodic macroscopic hematuria differs from the delayed-onset hematuria that appears 2 to 3 weeks after pharyngitis in PSGN. Macroscopic hematuria usually resolves spontaneously within days, and microscopic hematuria often occurs between mucosal infections.
Table 2 Diseases Associated with IgA Deposits
|
Dermatologic diseases |
IgA monoclonal |
|
Dermatitis herpetiformis |
gammopathy |
|
Psoriasis |
Mycosis fungoides |
|
Reiter syndrome |
Sezary syndrome |
|
Gastrointestinal diseases |
Rheumatic diseases |
|
Celiac disease |
Ankylosing spondylitis |
|
Crohn disease |
Rheumatoid arthritis |
|
Ulcerative colitis |
Unclassified |
|
Hepatic diseases |
Diabetes |
|
Alcoholic liver diseases |
Idiopathic pulmonary h Aci n frnci c |
|
Hepatic cirrhosis |
1IC111U51UC1U515 Properdin deficiency |
|
Neoplastic diseases |
Retroperitoneal sclerosis |
|
Bronchial carcinoma |
Sarcoidosis |
Figure 4 IgA immunostaining showing IgA deposition in a typical mesangial cell distribution in IgA nephropathy.
Asymptomatic microscopic hematuria and nonnephrotic-range proteinuria constitute 30% to 40% of IgA nephropathy; nephrotic syndrome constitute approximately 5%. Acute renal failure, which constitutes less than 5%, can occur in association with crescents (i.e., crescentic IgA nephropathy) or, possibly, as a result of acute tubular necrosis from excessive hematuria. In rare instances, IgA nephropathy presents as part of a systemic disease [see Table 2].
Laboratory investigations are usually not helpful in diagnosing IgA nephropathy. Serum IgA levels are increased in about a third of patients, and IgA-fibronectin aggregates are increased in about half. Mesangial deposition of C3 is found in more than half of patients. Serum complement levels are usually normal. Taken together, the diagnosis is clinical, supported by a renal biopsy.
The differential diagnosis of persistent isolated microscopic glomerular hematuria includes hereditary glomerulonephritis (Alport syndrome), postinfectious glomerulonephritis, thin basement membrane disease, SLE, FSGS, and minimal change disease. A major consideration in the differential diagnosis of IgA nephropathy is Henoch-Schonlein purpura (HSP). HSP is a systemic vasculitis that is characterized by deposition of IgA-containing immune complexes in tissues, including mesangial cells, and thus shares many features with IgA nephropathy.32 HSP is manifested clinically by a classic tetrad of purpuric rash (typically over the extremities), arthralgias (in knees and ankles), abdominal pain, and renal disease. Although HSP is more common in children younger than 5 years, renal involvement is more common in older children and adults. Like IgA nephropathy, HSP often follows an upper respiratory tract infection. A typical urinary finding is mild proteinuria and an active urinary sediment (containing RBCs and RBC casts), which is often transient. Most patients are asymptomatic, with a normal or slightly elevated serum creatinine level, but nephrotic syndrome develops in 20% of cases. The diagnosis of HSP can often be made clinically. Confirmation requires evidence of tissue deposition of IgA on either a skin biopsy (which shows a leukocytoclastic vasculitis) or renal biopsy (which shows an IgA immunostaining in a typical mesangial distribution). HSP is discussed in detail elsewhere [see 10:VII Vascular Diseases of the Kidney].
Figure 5 Light micrograph from a patient with IgA nephropathy showing the characteristic glomerular hypercellularity.
Treatment Although a variety of treatment strategies have been tried, therapy for IgA nephropathy remains disappoint-ing.33 Several studies suggest that the use of ACE inhibitors, even in normotensive patients, may slow progression by lowering glomerular filtration pressure and reducing proteinuria.34-36 Combining ACE inhibitors with ARBs may enhance the antiprotein-uric effect and further slow the progression of renal disease.37 In patients with aggressive or progressive IgA nephropathy, slowing or arresting of disease progression may be accomplished with the combination of cytotoxic agents (oral cyclophos-phamide, 1.5 mg/kg daily for 3 months, then converted to aza-thioprine) and prednisolone.38 Clinical trials evaluating the efficacy of fish oil have produced conflicting results, and a meta-analysis concluded that a minor benefit was likely.
All patients with IgA nephropathy should receive treatment with an ACE inhibitor, an ARB, or both. Corticosteroid therapy for 18 to 36 months may be associated with less proteinuria and better outcome.4043 However, studies showing a benefit from cor-ticosteroid therapy were performed before the current aggressive use of angiotensin antagonists. A subset of proteinuric patients without hematuria (usually with nephritic syndrome and minimal glomerular changes on light microscopy and efface-ment of the foot processes on electron microscopy) seem to respond very well to steroid treatment. Some experts recommend steroid treatment only for patients with nephrotic syndrome and minimal apparent changes on histology; progressive active disease despite the use of ACE inhibitors, ARBs, or both; or severe disease on biopsy.
Prognosis Most patients with IgA nephropathy have an indolent course, with chronic intermittent hematuria. Risk factors for disease progression include an elevated serum creatinine level and elevated blood pressure on presentation (although these may instead reflect severe disease), persistent proteinuria (> 1 g/day), older age, and interstitial fibrosis on renal biopsy. For reasons that are still unclear, recurrent macroscopic hematuria is often associated with a better prognosis than persistent microscopic hematuria and proteinuria. Serum IgA levels and gender are not prognostic factors. IgA nephropathy is a significant cause of end-stage renal failure, with 20% of patients requiring renal replacement therapy within 20 years of presentation.
Figure 6 Schematic view outlining the events in IgA nephropathy leading to mesangial hypercellularity and matrix expansion. (a) IgA deposition between mesangial cells is followed by (b) proliferation of mesangial cells, which leads to (c) mesangial hypercellularity and matrix expansion.