Calcium Metabolism
The precise regulation of body calcium stores and of the calcium concentration in both extracellular and intracellular compartments is critically important, for the following reasons: calcium is the chief mineral component of the skeleton; calcium serves major roles in neurologic transmission, muscle contraction, and blood coagulation; and it is a ubiquitous intracellular signal. A typical laboratory range for serum calcium concentration is between 8.8 and 10.5 mg/dl; 50% to 60% of the calcium in the blood is bound to plasma proteins or is complexed with citrate and phosphate. The remaining ionized (free) calcium controls physiologic actions. The body regulates not only ionized calcium concentrations but also the entry and exit of calcium into its main storage site, bone, through the activity of parathyroid hormone (PTH) and 1,25-dihydroxyvitamin D3 (1,25-(OH)2D3) [see Figure 1 ]. PTH, secreted by the parathyroid glands, is an 84-amino acid peptide with a very short plasma half-life (2 to 4 minutes). Cholecalciferol (vitamin D3) is generated by the skin, upon exposure to ultraviolet light; it is also supplied by dietary sources (chiefly fortified liquid milk products). In the liver, vitamin D3 is hydroxylated to 25-(OH)D3, which is in turn hy-droxylated in the kidney to 1,25-(OH)2D3 (calcitriol), markedly increasing its potency. In concert, this hormonal system expresses its action at the level of the gastrointestinal tract, bone, and the kidney and maintains circulating ionized calcium concentrations under extremely tight control (variation < 0.1 mg/dl), despite significant variations in calcium supply.
Figure 1 Circulating concentrations of ionized calcium are maintained under extremely tight control by parathyroid hormone (PTH) and the vitamin D axis. Absorption of dietary calcium by the gastrointestinal tract, reduction of calcium excretion by the kidneys, and release of stored calcium from bones serve as sources for circulating calcium. Decreases in circulating calcium trigger the release of PTH, which promotes release of calcium into the extracellular space by increasing bone resorption; the release of PTH also causes an increase in calcium reabsorption in the distal nephron, resulting in a decrease in urinary calcium loss. PTH also augments renal production of 1,25-dihydroxyvitamin D, which secondarily increases calcium absorption in the gut.
Under normal conditions, despite ranges in dietary calcium consumption that can vary from 400 to 2,000 mg daily, net calcium absorption from the GI tract averages about 150 to 200 mg/day. In steady state, this equals the amount of calcium excreted by the kidneys. Ongoing remodeling of bone results in the consumption and release of approximately 500 mg of calcium a day. Through humoral regulation, this calcium reservoir can be exploited to maintain extracellular calcium levels in a narrow range despite increased physiologic need or decreased intake, such as results from severe curtailment of the dietary calcium supply or from impairment of intestinal calcium absorption.
Changes in the extracellular ionized calcium concentration are registered by parathyroid cells via the cell surface calcium-sensing receptor (CaSR).1 Interaction of calcium ions with the extracellular domain of the CaSR triggers a series of intracellular signaling events, which ultimately govern PTH secretion. As circulating concentrations of calcium fall, PTH secretion rises, and vice versa.
PTH increases bone resorption and distal nephron calcium reabsorption, the former promoting calcium release into the extracellular space and the latter decreasing urinary calcium losses. PTH also augments renal production of calcitriol, which then increases fractional calcium absorption in the gut. If calcium intake increases beyond the body’s needs, PTH secretion decreases, leading to decreased calcitriol production and decreased calcium absorption by the gut. If calcium is absorbed in excess of requirements, it will be promptly excreted. In this elegant manner, circulating ionized calcium concentration is guarded closely, albeit sometimes at the expense of skeletal calcium stores. Disturbances of PTH, vitamin D action, or both are most often manifested by altered serum calcium or phosphate concentration and by abnormal bone turnover. In some cases, bone mineral density (BMD) is decreased.
Measurement of Calcium
Diagnosis of a calcium disorder depends first on accurate measurement of serum or ionized calcium or both. Serum measurements are usually performed by spectrophotometry or by atomic absorption spectrophotometry, which yields more accurate measurements. Spurious readings may occur with tourniquet stasis (i.e., if the tourniquet is in place too long before the blood is drawn), which can elevate serum calcium values by up to 1 mg/dl. Dilution of blood by drawing samples from indwelling intravenous catheters is a common error that leads to spuriously low calcium readings. Ionized calcium measurements should be considered accurate only when performed on samples collected anaerobically (i.e., in a blood gas syringe) and placed on ice, with immediate analysis.
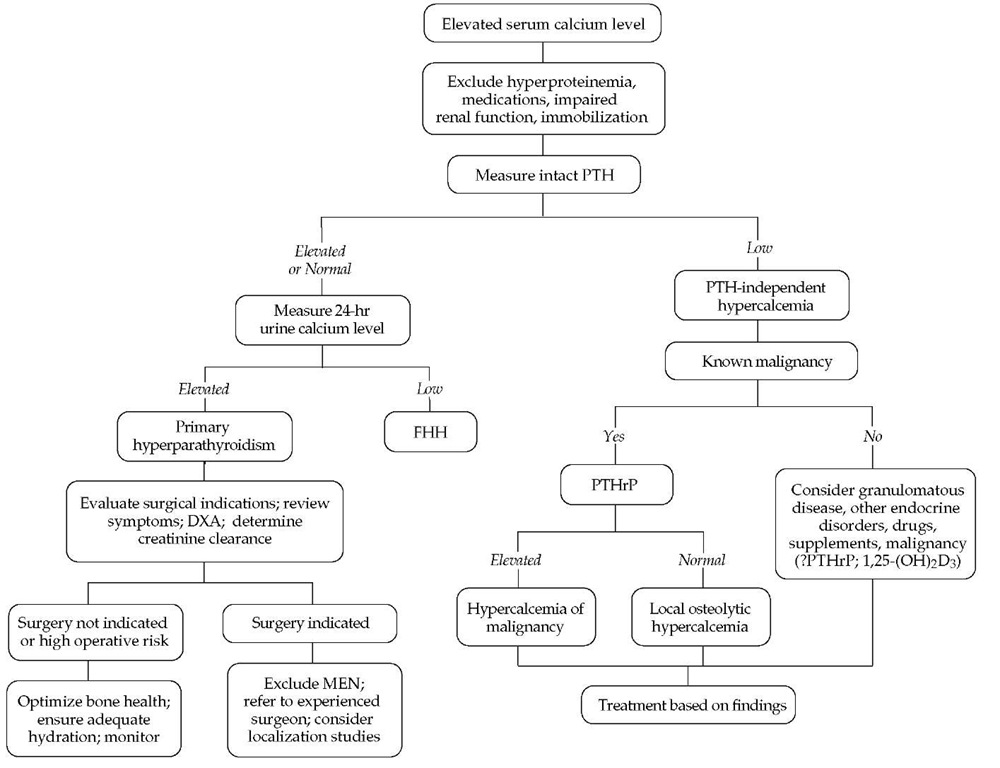
Figure 2 Evaluation and management of hypercalcemia. (DXA—dual-energy x-ray absorptiometry; FHH—familial hypocalciuric hypercalcemia; MEN—multiple endocrine neoplasia; PTH—parathyroid hormone; PTHrP— parathyroid hormone-related protein)
Table 1 Differential Diagnosis of Hypercalcemia
|
Parathyroid hormone-mediated hypercalcemia |
Primary hyperparathyroidism Parathyroid adenoma Parathyroid hyperplasia Parathyroid carcinoma Tertiary hyperparathyroidism |
|
Humoral hypercalcemia of malignancy |
|
|
Parathyroid hormone-related protein |
|
|
mediated |
|
|
Squamous cell carcinoma of the lung |
|
|
Carcinoma of the oropharynx, |
|
|
nasopharynx, larynx, and esophagus |
|
|
Cervical carcinoma |
|
|
Ovarian carcinoma |
|
|
Renal cell carcinoma |
|
|
Transitional cell carcinoma of the |
|
|
bladder |
|
|
Pheochromocytoma |
|
|
Islet cell neoplasms of the pancreas |
|
|
T cell lymphoma |
|
|
Others |
|
|
1,25-(OH)2 D3 mediated |
|
|
B cell lymphoma |
|
|
Local osteolytic hypercalcemia |
|
|
Parathyroid hormone-independent hypercalcemia |
Multiple myeloma |
|
Breast carcinoma |
|
|
Lymphoma |
|
|
Others |
|
|
Medications/supplements |
|
|
Vitamin D |
|
|
Vitamin A |
|
|
Lithium |
|
|
Thiazides |
|
|
Calcium antacids (milk-alkali |
|
|
syndrome) |
|
|
Granulomatous diseases |
|
|
Sarcoidosis |
|
|
Tuberculosis |
|
|
Histoplasmosis |
|
|
Leprosy |
|
|
Other conditions |
|
|
Increased plasma protein levels (factitious |
|
|
hypercalcemia) |
|
|
Acute renal failure |
|
|
Thyrotoxicosis |
|
|
Adrenal insufficiency |
|
|
Immobilization |
|
|
Familial hypocalciuric hypocalcemia |
|
|
(benign familial hypercalcemia) |
Hypercalcemia
Hypercalcemia is a common metabolic abnormality. Signs and symptoms of hypercalcemia vary significantly from patient to patient and correlate somewhat with the degree of calcium elevation and its rate of change. The diagnostic workup of hyper-calcemia is straightforward [see Figure 2].2 The etiology of hyper-calcemia is usually discovered after a comprehensive history, physical examination, focused laboratory assessment, and, occasionally, diagnostic imaging studies.3
Clinical manifestations
Most patients with mild hypercalcemia (serum calcium level < 11 mg/dl) are asymptomatic, although some may experience mild fatigue, vague changes in cognitive function, depression, or polyuria and polydipsia (from decreased urine concentrating ability caused by a high calcium level). Those with moderate hy-percalcemia (serum calcium levels of 11 to 14 mg/dl) are more likely to be symptomatic. The likelihood of classic manifestations of hypercalcemia increases sharply when calcium levels rise to 12 to 14 mg/dl. These symptoms include anorexia, nausea, vomiting, abdominal pain, constipation, muscle weakness, and altered mental status. Severe hypercalcemia (i.e., serum calcium levels greater than 14 mg/dl) may cause progressive lethargy, disorientation, and even coma.
In addition to the degree of elevation, the rate of increase in serum calcium may influence the clinical picture. Chronically hypercalcemic patients can function and feel reasonably well with serum calcium values even as high as 15 to 16 mg/dl. In contrast, patients whose calcium level has risen abruptly will often experience symptoms at lesser calcium elevations. Elderly or debilitated patients are also more likely to be symptomatic.
History and physical examination
The history and physical examination are directed at uncovering signs or symptoms of hypercalcemia, as well as signs of the most common causes of hypercalcemia: hyperparathy-roidism, malignancy, granulomatous diseases, and certain en-docrinopathies. Evidence of any related condition, such as osteoporosis or urinary tract stones, should also be sought. The medical record should be reviewed to determine the duration of the hypercalcemia. The most common cause of hypercalcemia, primary hyperparathyroidism, presents as stable or gradually progressive elevation of the serum calcium level over a period of years. In contrast, malignancy typically causes a more acute rise in serum calcium. All recent medications, foods, and nutritional supplements should be thoroughly reviewed for possible culprits. A careful family history should be performed to identify disorders of calcium metabolism; renal stones; fragility fractures; and any related endocrinopathies, such as diseases of the pituitary, adrenal, thyroid, or endocrine pancreas.
Aside from mental status deficits and signs of dehydration, physical examination findings are generally normal in patients with hypercalcemia, especially if calcium levels are only mildly to moderately elevated. Rarely, severe and prolonged hypercal-cemia results in a visible horizontal calcium deposit on the cornea, a condition known as band keratopathy. Other signs and symptoms depend on the etiology of the elevation [see Table 1 ]. Patients with hyperparathyroidism classically have osteope-nia, bone pain, or nephrolithiasis. Currently, however, most cases of primary hyperparathyroidism are identified before the patient becomes symptomatic. Patients whose hyperparathy-roidism is associated with multiple endocrine neoplasia (MEN) syndromes may have specific manifestations of the other conditions that are part of these syndromes. Patients with sarcoidosis may present with fever, lymphadenopathy, skin rashes, or pulmonary symptoms. Hypercalcemia of malignancy develops only when a substantial tumor burden is present; consequently, most of these patients have an established cancer diagnosis and clinical features associated with the specific tumor type and extent of disease.
Laboratory studies
The first step in the laboratory assessment is to exclude factitious hypercalcemia, which may result from an increase in circulating concentrations of plasma proteins. About 50% to 60% of circulating calcium is bound to these proteins, so elevation in their concentrations (as occurs in HIV infection, chronic viral hepatitis, and multiple myeloma) will produce a proportionate rise in the total calcium concentration. The ionized calcium concentration, however, remains normal. To adjust for elevations in plasma protein, the serum calcium level should be lowered by 0.8 mg/dl for every 1 g/dl of albumin (or protein) above the normal range. When performed correctly, ionized calcium measurement is more accurate than adjusted total calcium. Because acute renal failure may occasionally lead to hypercalcemia, renal function should also be assessed.
Once hypercalcemia is confirmed, the next step is measurement of the serum PTH concentration. This is the most important test for determining the cause of hypercalcemia.3 Several PTH assays are commercially available. The most commonly utilized is the two-site immunochemiluminometric assay (ICMA, or so-called bio-intact PTH). Earlier assays could not distinguish between full-length PTH and inactive molecular fragments that circulate in significant concentrations. The ICMA measures only the intact PTH molecule and is therefore the preferred test in most instances, especially in patients whose serum creatinine level is elevated.
Other helpful tests include measurement of serum creatinine and alkaline phosphatase, as well as inorganic phosphorus assays and an electrolyte panel. Assessment of 24-hour urinary calcium excretion is usually performed. Serum creatinine may be elevated in patients with nephrocalcinosis secondary to prolonged hypercalcemia. The alkaline phosphatase level may be elevated in patients with hypercalcemic states involving increased bone turnover. Patients with hypercalcemia caused by malignancy may demonstrate biochemical or hematologic findings consistent with the site of neoplasia and the degree of its dissemination. Most causes of hypercalcemia are also accompanied by hypercalciuria (24-hour urinary calcium excretion > 4 mg/kg/day), which may lead to nephrocalcinosis or renal stone formation. A serum calcium x phosphate product greater than 70 suggests the patient is at risk for calciphylaxis, and efforts to lower the serum phosphate level (e.g., with phosphate binders) should accompany the interventions to lower serum calcium.
Other diagnostic studies may be dictated by clinical circumstances. Electrocardiographic abnormalities of severe hypercal-cemia include shortening of the QTc interval and, rarely, atrio-ventricular blocks. In addition, many hypercalcemic conditions cause a decrease in BMD, which may be noted on plain x-rays but is best quantified by measurement of bone density (see below). Abdominal x-rays may identify renal stones or nephrocal-cinosis. Specific bone radiographic findings are few, and in primary hyperaparathyroidism, specific bony abnormalities are now rare, thanks to early detection of hypercalcemia.
Differential diagnosis
The results of PTH measurement indicate whether hypercal-cemia is or is not mediated by PTH and thus provide a broad indication of the cause of hypercalcemia [see Table 1]. When PTH levels are high or, in some cases, inappropriately normal, the hy-percalcemia is PTH mediated; this is commonly referred to as hy-perparathyroidism. When PTH levels are suppressed, the hyper-calcemia is said to be non-PTH mediated, or PTH independent. In turn, this distinction guides subsequent patient assessment.