Definition and Overview
Diabetes mellitus is a metabolic disease characterized by hyperglycemia that results from defects in insulin secretion, insulin action, or both. Important abnormalities in fat and protein metabolism are also present. Nonetheless, the diagnosis still rests upon demonstrating elevated plasma glucose levels. The chronic hyperglycemia of diabetes mellitus is specifically associated with long-term damage, dysfunction, and failure of various organs, especially the retina and lens of the eye, the kidneys, and both somatic and autonomic nervous systems. The heart, arterial system, and microcirculation are also adversely affected.
A variety of pathogenic processes are involved in the development of different forms of diabetes. These processes range from autoimmune destruction of the beta cells of the pancreatic islets with consequent insulin deficiency to mutations in the insulin receptor gene with consequent resistance to insulin action. The basis for the metabolic abnormalities of diabetes mellitus is deficient action of insulin on its major target tissues, including skeletal muscle, cardiac muscle, adipose tissue, and liver. Loss of proper insulin regulation of metabolism results from inadequate secretion of insulin, from diminished tissue responses to insulin at one or more points in the complex pathways of insulin action, or from both processes. Impairment of insulin secretion and defects in insulin action coexist in many patients, and in these patients, it is often unclear which abnormality is the primary cause of the hyperglycemia.
Acute life-threatening consequences of diabetes mellitus are ketoacidosis and nonketotic hyperglycemic hyperosmolar coma. Overtreatment of hyperglycemia can lead to hypoglycemia, which may be severe enough to cause seizures and loss of consciousness. Symptoms of poorly controlled hyperglycemia include polyuria, polydipsia, blurred vision, weight loss, polypha-gia, stunting of growth, and vulnerability to infections or susceptibility to a more virulent or chronic course when infected.
Specific long-term complications of diabetes include (l) retinopathy with potential loss of vision, (2) nephropathy leading to end stage renal disease (ESRD), and (3) neuropathy with risk of foot ulcers, amputation, Charcot joints, sexual dysfunction, and potentially disabling dysfunction of the stomach, bowel, and bladder. Numerous mechanisms have been discovered that may mediate the specific tissue damage caused by hyper-glycemia. Diabetic patients are also at increased risk for atherosclerotic cardiovascular, peripheral vascular, and cerebrovascu-lar disease. These conditions may be related to hyperglycemia as well as to hypertension and abnormal lipoprotein profiles that are often found in diabetic patients.
Sufficient hyperglycemia to cause pathologic and functional changes in target tissues may be present for some time before clinical symptoms lead to a diagnosis of diabetes in many patients. At an even earlier stage, an incipient abnormality in glucose metabolism can be identified on plasma glucose testing, which indicates that the patient is at considerably increased risk for the full clinical disorder.
Classification
The classification of diabetes mellitus has recently been revised by a task force of the American Diabetes Association that included representation from Europe.1 Major etiologic classes of the disease, along with more esoteric examples, have been categorized [see Table 1]. The vast majority of cases of diabetes mellitus are either type 1 (insulin-dependent) or type 2 ( non-insulin-dependent) in an approximate ratio of 1:9.
Type 1 and type 2 diabetes mellitus
Type 1 and type 2 diabetes were formerly known as insulin-dependent diabetes mellitus (IDDM) and non-insulin-dependent diabetes mellitus (NIDDM), respectively. This classification was abandoned largely because it was difficult to distinguish patients with IDDM from those patients with NIDDM who eventually required insulin treatment to mitigate hyperglycemia. Physicians, nurses, hospital-record-room personnel, health insurers, and even sometimes researchers were hard put to distinguish between these two forms of diabetes using the old terminology. The new classification, dependent on etiology rather than mode of treatment, puts a greater emphasis on the history and characteristics of the patients to determine the probable etiology and type. Two categories of blood glucose elevation, impaired glucose tolerance (IGT) and impaired fasting glucose (IFG), that lie between normal glucose levels and overt diabetes have also been established [see Impaired Glucose Tolerance, below].2
Gestational diabetes mellitus
Gestational diabetes mellitus (GDM) constitutes a separate category for cases of diabetes first detected during pregnancy.3 When diabetes is detected early in pregnancy, it is likely to be type 1 or type 2 diabetes mellitus that is presenting symptomatically and was probably precipitated or worsened by the pregnant state. Diabetes is commonly detected in the second and third trimester (i.e., in 4% of pregnant women) and is likely to be specific for the pregnant state, to be transient, and to reverse to normal glucose tolerance or to IGT on follow-up oral glucose tolerance testing 6 weeks after delivery. However, GDM is associated with a high risk of future diabetes, especially in women who have IGT post partum or who remain obese.3 Permanent diabetes will develop in approximately 50% of patients within 10 years of GDM. The greatest importance of any single episode of GDM lies in the risks it poses to the fetus. These risks include intrauterine mortality, neonatal mortality, respiratory distress syndrome, hypoglycemia, hypocalcemia, jaundice, and macrosomia, which can cause trauma such as shoulder dystocia during passage through the birth canal.
Secondary forms of diabetes mellitus
Of the categories of secondary diabetes [see Table 1], endo-crinopathies and drug- or chemical-induced diabetes are noteworthy because they represent instances of diabetes that are potentially reversible if they are recognized and the physician can cure the endocrinopathy or discontinue the offending drug. The category of genetic defects in beta cell function illustrates how the classification will grow ever more detailed as knowledge increases. For example, the single diabetes mellitus phenotype formerly called maturity-onset diabetes of the young (MODY) can now be more precisely classified into at least four genetic varieties, each of which arises from mutation of a different gene.
Diabetes caused by chronic pancreatitis, pancreatectomy, or occasionally carcinoma of the pancreas is usually type 1 in character. Because patients with this disease have glucagon as well as insulin deficiency, they are somewhat less likely to go into ke-toacidosis4 but are quite vulnerable to hypoglycemia. Because they are deficient in pancreatic enzymes, their digestion and subsequent absorption of nutrients is somewhat erratic, even though replacement enzymes are ingested with meals. If alcoholism, often the cause of chronic pancreatitis, is irremediable, it also contributes to blood glucose instability, as does the often accompanying irregular lifestyle. Small frequent doses of lispro insulin should be helpful, but safety may require less stringent blood glucose goals in such patients.
Although many individual drugs have been incriminated as a cause of hyperglycemia, the continued use of pharmacologic anti-inflammatory or immunosuppressive doses of synthetic gluco-corticoids is an especially important continuing problem. Up to 25% of renal transplant patients develop so-called steroid dia-betes.5 In a case-control study, use of glucocorticoids for up to 45 days was a risk factor for diabetes that required pharmacologic treatment.6 The odds ratio rose from 1.77 at a prednisone equivalent of 10 mg/day to an odds ratio of 10.3 at 30 mg/day. Obesity and family history of diabetes increased the risk of steroid diabetes. Although insulin resistance in the liver and muscle is a well-recognized effect of glucocorticoids, an action on the beta cells to limit the compensatory response to hyperglycemia7 adds to the diabetogenic effect at higher steroid doses. Patients treated with glucocorticoids for more than a few days need to be warned to watch for and report clinical symptoms of hyperglycemia promptly. Ketoacidosis is rare, but hyperglycemic hyperosmolar nonketotic coma can occur. Insulin treatment is usually necessary for symptomatic patients and for those with a fasting plasma glucose (FPG) level greater than 200 mg/dl, but sulfonylurea drugs are sometimes effective. There is little systematic information on the efficacy of the other oral agents. In most instances, steroid diabetes is transient, but in a minority of cases, diabetes persists even after withdrawal of the glucocorticoids.
|
Table 1 Etiologic Classification of Diabetes |
|
Type 1 diabetes mellitus* (3 cell destruction, usually leading to absolute insulin deficiency) |
|
Immune mediated |
|
Idiopathic |
|
Type 2 diabetes mellitus* (may range from predominantly insulin resistance with relative insulin deficiency to a predominantly insulin secretory defect with insulin resistance) |
|
Other specific types of diabetes |
|
Genetic defects of 3 cell function |
|
Chromosome 12, HNF-1a (formerly MODY3) |
|
Chromosome 7, glucokinase (formerly MODY2) |
|
Chromosome 20, HNF-4a (formerly MODY2) |
|
Genetic defects in insulin action |
|
Type A insulin resistance |
|
Disease of the exocrine pancreas |
|
Pancreatitis |
|
Trauma/pancreatectomy |
|
Neoplasia |
|
Endocrinopathies |
|
Acromegaly |
|
Cushing syndrome |
|
Glucagonoma |
|
Drug- or chemical-induced |
|
Nicotinic acid |
|
Glucocorticoids |
|
Thiazides |
|
Infections |
|
Congenital rubella |
|
Cytomegalovirus |
|
Uncommon forms of immune-mediated diabetes |
|
Stiff-man syndrome |
|
Anti-insulin receptor antibodies |
|
Other genetic syndromes associated with diabetes |
|
Down syndrome |
|
Turner syndrome |
|
Friedreich ataxia |
|
Myotonic dystrophy |
|
Gestational diabetes mellitus (GDM) |
Note: The list of other specific types of diabetes is not comprehensive. There are many other such syndromes.
*Patients with any form of diabetes may require insulin treatment at some stage of their disease. Such use of insulin does not, of itself, classify the patient.
Screening for Diabetes
Screening for type 1 diabetes mellitus by office glucose testing is currently indicated in high-risk patients. Current American Diabetes Association criteria for office screening of asymptomatic individuals for type 2 diabetes mellitus employ FPG levels.1 Screening is recommended in all individuals 45 years of age and older at 3-year intervals. Younger individuals should be screened if they are obese (> 120% desirable body weight or a body mass index > 27), have a first-degree relative with diabetes, are members of a high-risk ethnic population (African American, Hispanic American, Native American, Asian American), have delivered a baby weighing more than 9 lb, have previously had GDM, are hypertensive (blood pressure > 140/90 mm Hg), have atherogenic dyslipidemia (high-density lipoprotein [HDL] cholesterol levels < 35 mg/dl or triglyceride levels > 250 mg/dl) or had IFG or IGT on previous testing.1 Mass indiscriminate public screening is not justified, because there is as yet no proof of population benefit.
Epidemiology
Type 1 diabetes mellitus
Available, but not up-to-date, studies suggest the prevalence of type 1 diabetes mellitus in the United States is 1.7 per 1,000 in individuals younger than 19 years and 2.1 per 1,000 in adults.8 A total prevalence of approximately 500,000 is estimated. Current estimates of annual incidence are 18 per 100,000 population in the 0- to 19-year age range and 9 per 100,000 population in those older than 20 years.8 Approximately 30,000 cases of type 1 diabetes mellitus are estimated to occur yearly in the United States, and it is more common in whites than in African Americans. Worldwide, the highest annual incidence of type 1 diabetes mellitus is found in Finland (35 cases per 100,000) and the lowest is found in Korea (< 1 per 100,000).
Type 2 diabetes mellitus
Analysis of data from the third National Health and Nutrition Examination Survey (NHANES III), conducted from 1988 to 1994,9 indicates a prevalence of 5.1% for adults at least 20 years of age in the United States and a prevalence of 2.7% of undiagnosed diabetes (FPG > 126 mg/dl). A prevalence of 12.3% (diagnosed plus undiagnosed) was estimated for individuals 40 to 74 years of age. There are an estimated 10.2 million diagnosed and 5.4 million undiagnosed cases of diabetes in the United States. The estimated number of persons with IGT approximately equals the number with diabetes. Non-Hispanic African-American and Mexican-American women have nearly twice the prevalence of diabetes as non-Hispanic white women. Non-Hispanic African-American men have a slightly higher risk than non-Hispanic white men, but Mexican-American men have about a 50% greater risk than non-Hispanic white men.
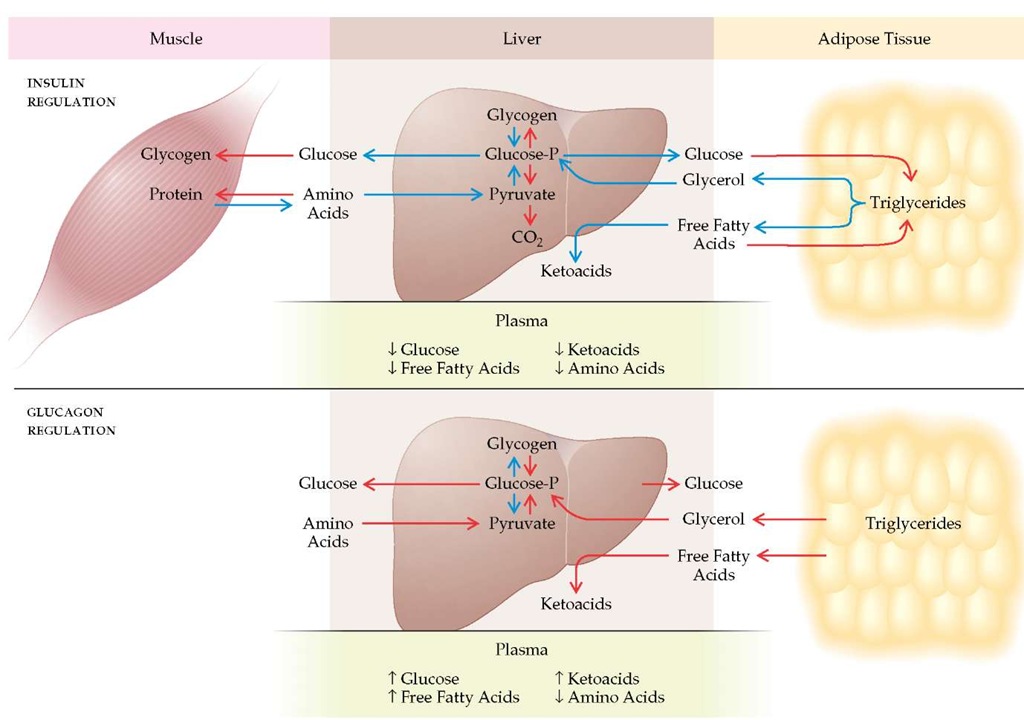
Figure 1 The opposing actions of insulin and glucagon, particularly within the liver, on substrate flow and plasma levels are seen here. The two hormones have directly opposite effects on key enzymes, such as glycogen synthase and phosphorylase. Thus, stimulatory effects of glucagons on glucose and ketoacid production are magnified when insulin is deficient, as in type 1 diabetes mellitus. Red arrows indicate stimulation. Blue arrows indicate inhibition.
Annual incidence of type 2 diabetes mellitus per 100,000 population ranges from 180 in 25 to 44 year olds to a peak of 860 in 65 to 74 year olds. Approximately 625,000 cases of type 2 diabetes mellitus develop yearly in the United States.10 The prevalence is expected to rise from 15 million in the year 2000 to 21 million in 2025. Worldwide, the prevalence of type 2 diabetes mellitus will likely increase from 150 million to 300 million during that time.11 The increase42 reflects aging of the population, strikingly increased obesity,13 and a sedentary lifestyle. This rise in the number of cases is especially troubling in regard to high-risk ethnic minorities whose access to medical care may be limited.14,15 Obesity is a major risk factor for type 2 diabetes mellitus.16 The current definition of obesity employs the body mass index (BMI) (body weight in kilograms divided by height in meters squared).
A person with a BMI of at least 25 but less than 30 is defined as overweight.17 A BMI of 30 or more is defined as obesity,16 and a BMI of 40 and above is associated with a 15-fold increased risk of type 2 diabetes mellitus.15 Abdominal obesity, defined as a waist circumference greater than 100 cm in men and greater than 88 cm in women or a waist-to-hip ratio greater than 0.9, is an especially strong risk factor for type 2 diabetes mellitus. A large preponderance of patients with type 2 diabetes mellitus are obese; even those with normal BMI may have an increased percentage of their body weight accounted for by fat.18 Longer duration of obesity further increases the risk of diabetes, emphasizing the importance of early efforts to control weight. Many patients with type 2 diabetes mellitus have a strong family history of that disease in first-degree relatives. An extraordinary example is found among the Arizona Pima Indians on the Gila River reservation, where 50% of the adult population has type 2 diabetes mellitus. Other risk factors for the disease include physical inactivity, hypertension, dyslipidemia, gestational diabetes, low birth weight, low income, low level of education, and low socioeconomic status.
Hormonal Regulation of Metabolism
Diabetes involves the most fundamental aspects of human metabolism. The following are all affected by the hormonal abnormalities of diabetes: energy production and expenditure; the proportioning of carbohydrate, fat, and protein as energy sources; the storage of energy as carbohydrate and fat; and the balance between protein synthesis (anabolism) and degradation (catabo-lism). To understand the pathogenesis of diabetes, it is useful to start with a brief review of normal metabolism.
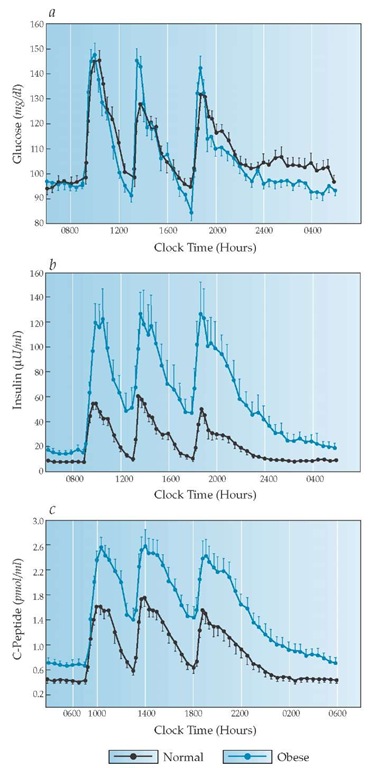
Figure 2 Plasma glucose (a) is normally kept within a narrow range throughout the day, largely because of beta cell function. Plasma insulin (b) and plasma C-peptide (c) rise sharply from their basal levels with each meal and, after reaching peaks, return promptly to basal levels, which are maintained throughout the night. Note also that plasma insulin and C-peptide levels are elevated in obese individuals who are insulin resistant.
A proper balance between insulin and glucagon is one crucial hormonal regulator of basal metabolic homeostasis.20 Insulin primarily facilitates storage of glucose as glycogen, free fatty acids in triglycerides, and amino acids in protein, and it inhibits glycogenolysis, lipolysis, ketogenesis, proteolysis, and gluconeogenesis [see Figure 1]. Glucagon stimulates mobilization of glucose, free fatty acids, and glycerol and stimulates hepatic uptake of amino acids and the conversion of their carbon skeletons to glucose. Glucagon also stimulates ketogenesis from free fatty acids. The normal steady-state levels of insulin and glucagon help maintain the overnight FPG level at 60 to 110 mg/dl, free fatty acid levels at less than 0.7 mmol/L, ketoacids at less than 0.2 mmol/L, and each amino acid at its unique level. After a mixed meal, plasma insulin rises sharply [see Figure 2] and, with it, the insulin-glucagon ratio. This condition reverses all the previously described processes. Dietary carbohydrate is stored in muscle and liver glycogen, free fatty acids are reesteri-fied and stored as triglycerides in adipose tissue, and protein metabolism shifts back toward anabolism. When all the nutrients have been assimilated and plasma glucose returns to its basal preprandial level, plasma insulin [see Figure 2] and the in-sulin-glucagon ratio promptly return to basal levels, preventing an overshoot of insulin action that would otherwise cause hy-poglycemia. Thus, an immediate rise, an early peak, and a prompt fall in insulin secretion are requisite to normal postprandial metabolism [see Figure 2].
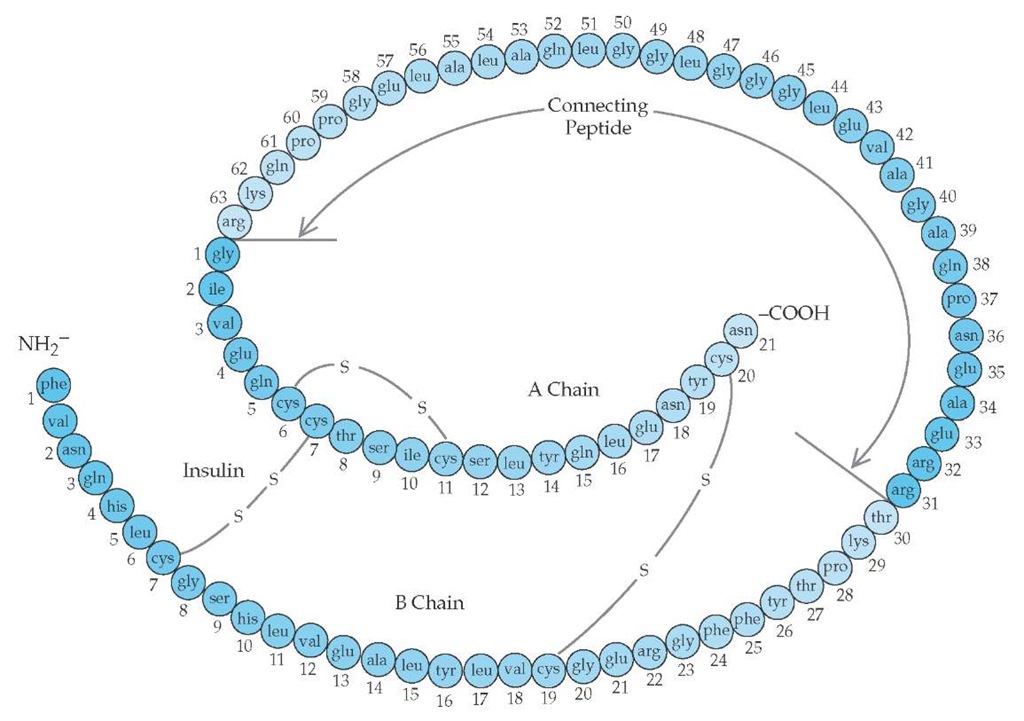
Insulin is synthesized in pancreatic islet beta cells from a larger molecule called proinsulin, which is then split to yield insulin and an intramolecular connecting peptide called C-peptide [see Figure 3]. The two molecules are stored in the same granules and secreted in an equimolar ratio when the beta cell is stimulated. Thus, plasma C-peptide levels are a faithful marker of beta cell function [see Figure 3].
Insulin acts via a plasma insulin receptor that leads to the generation of multiple mediators of insulin’s numerous intracellular cytoplasmic and nuclear effects [see Figure 4]. Insulin regulates both the activities and syntheses of target enzymes. Sensitivity of target tissues to insulin is the other major determinant of insulin action. Insulin sensitivity is best measured in humans by infusing insulin to establish steady-state plasma insulin levels [see Figure 5]. Simultaneously, the baseline plasma glucose is maintained at a constant level by a variable glucose infusion. The amount of glucose required to prevent plasma glucose from decreasing under the effect of insulin is equal to the increased amount of glucose being used per unit time under insulin stimulation (assuming that insulin has completely suppressed hepatic glucose output by the liver). The quantity of glucose used per unit time divided by the plasma insulin level provides an index of whole body sensitivity to insulin in the sphere of glucose metabolism.
A feedback loop exists between insulin responsiveness in target tissues and insulin secretion by beta cells. This relation operates to increase insulin secretion in individuals relatively resistant to insulin action and to decrease insulin release in individuals very sensitive to insulin action. The result is one critical mechanism for maintaining fasting and postprandial plasma glucose levels within narrow normal ranges.
Pathogenesis of Microvascular Complications in Diabetes
A distinctive feature of diabetes—the microvascular complications—were only revealed or commonly appreciated after the introduction of insulin therapy in 1922 allowed patients with type 1 diabetes mellitus to live long enough to experience these complications. It should be borne in mind that the descriptions and pathogenetic sequences presented below reflect a former commonly practiced degree of metabolic control no longer considered acceptable. Prevention of these complications is a major goal of current therapeutic policy and recommendations for all but transient forms of diabetes [see Prevention and Treatment of Microvascular Complications, below].
Figure 3 The structure of human proinsulin, the precursor molecule to insulin. The peptide that connects the amino terminus (NH2-) of the A chain to the carboxyl terminus (-COOH) of the B chain is called connecting peptide (C-peptide). Proinsulin is converted to insulin and C-peptide, and these two molecules are packaged together in the secretory granule. On stimulation of the beta cell, C-peptide and insulin are secreted in equimolar proportions. Thus, C-peptide levels reflect beta cell functional capacity.