Blistering Eruptions
Simple eruptions Fixed Drug Eruptions
Fixed drug eruptions usually appear as solitary pruritic, ery-thematous, bright-red or dusky-red macules that may evolve into an edematous plaque [see Figure 2]. In some patients, multiple lesions may be present. Blistering and erosion may occur on mucosal surfaces.
Figure 2 This 28-year-old man taking tetracycline for acne vulgaris developed a fixed drug eruption.
Fixed drug eruptions recur in the same skin area after read-ministration of the causative medication. Many drugs have been implicated in fixed drug eruptions, including phenolph-thalein, ibuprofen, sulfonamides, tetracyclines, and barbitur-ates.47 The pathogenesis of fixed drug eruptions has not been fully elucidated. A haplotype linkage in the setting of trimetho-prim-sulfamethoxazole-induced fixed drug eruptions was recently documented.48
Fixed drug eruptions are most common on the genitalia and in the perianal area, although they can occur anywhere on the skin surface. The onset of a fixed drug eruption can be sudden, developing within 30 minutes to 8 to 16 hours after ingestion of the medication. In patients who continue to take the offending drug, the number of eruption sites may gradually increase.
After the initial acute phase, which lasts days to weeks, residual hyperpigmentation develops. Some patients may complain of burning or stinging on the affected skin sites. Systemic manifestations, which are present in approximately 25% of cases, can include fever, malaise, and abdominal symptoms.48
No conclusive diagnostic tests are available, but a challenge or provocation test with the suspected drug may be useful in confirming the diagnosis. Patch testing at the site of a previous lesion yields a positive response in up to 43% of patients. Prick and intradermal skin tests are reported to yield positive reactions in 24% and 67% of patients, respectively, but results vary with different drugs and reaction patterns. Patients with macu-lopapular rashes are more likely to have positive patch tests than patients with urticarial rashes.49
Treatment includes discontinuance of the causative agent and symptomatic therapy (e.g., topical corticosteroids).
Pseudoporphyria
Pseudoporphyria is a cutaneous phototoxic disorder that can resemble either porphyria cutanea tarda (PCT) or erythro-poietic protoporphyria (EPP). Tetracycline, furosemide, and naproxen have been implicated in PCT- and EPP-pseudopor-phyria.50 The eruption may begin within 1 day after initiation of therapy or may be delayed for as long as 1 year. PCT-pseudo-porphyria is characterized by skin fragility, blister formation, and scarring in areas exposed to sunlight; it occurs with normal porphyrin metabolism.
Figure 3 Pemphigus foliaceus developed in this 64-year-old man taking enalapril.
Figure 4 Pemphigus vulgaris developed in this 59-year-old woman who took penicillamine as treatment for rheumatoid arthritis.
The second clinical pattern mimics EPP and presents as cutaneous burning, erythema, vesiculation, angular scars, and waxy thickening of the skin.
Because of the risk of permanent facial scarring, the implicated drug should be discontinued when skin fragility, blistering, or scarring occurs. In addition, broad-spectrum sunscreen and protective clothing should be recommended to the patient.
Complex eruptions
Drug-Induced Linear IgA Disease
Linear IgA disease is an autoimmune bullous dermatosis that is identified on the basis of the linear deposition of IgA at the basement membrane zone.51 This disease can be induced by such drugs as vancomycin, lithium, diclofenac, and amio-darone. The drug-induced disease probably represents an im-munologic response to the offending drug.
Drug-induced linear IgA disease is heterogeneous in clinical presentation. Cases have shown morphologies resembling erythema multiforme, bullous pemphigoid, and dermatitis herpeti-formis. Drug-induced disease cannot be distinguished from the idiopathic variety either clinically, histologically, or immuno-logically; however, the clinical courses of these presentations differ. In drug-induced disease, spontaneous remission occurs once the offending agent is withdrawn; in idiopathic linear IgA disease, immune deposits disappear from the skin once the lesions resolve. Steroids and dapsone do not influence the healing process in drug-induced disease, whereas these agents have proved effective in treatment of idiopathic linear IgA disease.52
Drug-Induced Pemphigus
Pemphigus may be drug induced or drug triggered (i.e., the latent disease is unmasked by the drug exposure).
Drugs that cause pemphigus are penicillin, rifampin, phenyl-butazone, propranolol, progesterone, piroxicam, interferon beta, interleukin-2, and levodopa.53 An active amide group found in masked thiol drugs such as penicillin and cephalosporins and in nonthiol drugs such as enalapril may contribute to the patho-genesis of pemphigus.53,54 Pemphigus foliaceus [see Figure 3] caused by penicillamine and other thiol drugs tends to resolve spontaneously in 35% to 50% of cases.53 The average interval to onset is 1 year. Antinuclear antibodies are detected in 25% of affected patients.
Nonthiol drug-induced pemphigus manifests clinical, histo-logic, immunologic, and evolutionary aspects similar to those of idiopathic pemphigus vulgaris [see Figure 4]. Drug-induced pemphigus is associated with mucosal involvement. Spontaneous recovery after drug withdrawal occurs in 15% of affected patients.
Treatment of drug-induced pemphigus begins with drug withdrawal. Systemic corticosteroids are often required until all symptoms of active disease disappear. Vigilant follow-up is required after remission for an early relapse to be detected. The patient’s serum should be monitored regularly for autoantibodies.
Erythema Multiforme, Stevens-Johnson Syndrome, and Toxic Epidermal Necrolysis
The eruptions of erythema multiforme (EM), SJS, and TEN may represent variants of the same disease process. Reactions encompass a spectrum ranging from the less serious eruptions seen in EM to more serious reactions seen in SJS and TEN [see Figure 5].
A large percentage of EM and SJS cases are not drug related and may develop after a variety of predisposing factors, including infections, neoplasia, and autoimmune diseases. The drugs most frequently cited as causes of EM, SJS, and TEN are anticonvulsants, antibiotics (e.g., sulfonamides), allopurinol, and NSAIDs (e.g., piroxicam).55 With anticonvulsants, risk appears to be greatest during the first 8 weeks of therapy.56
Figure 5 This 50-year-old woman developed toxic epidermal necrolysis 17 days after starting phenytoin therapy.
Figure 6 Acute generalized exanthematous pustulosis (small nonfollicular pustules on a red base) in a 70-year-old man who took cloxacillin as treatment for cellulitis.
The pathogenesis of severe cutaneous ADRs is unknown, although a metabolic basis has been hypothesized. Sulfonamides and anticonvulsants, the two groups of drugs most frequently associated with SJS and TEN, are metabolized to toxic metabolites that are subsequently detoxified in most persons. However, in predisposed patients with a genetic defect, the metabolite may bind covalently to proteins. In some of these patients, the metabolite-protein adducts may trigger an immune response that leads to a cutaneous ADR.57
Clinically, the reaction patterns of EM, SJS, and TEN are characterized by the triad of mucous membrane erosions, target lesions, and epidermal necrosis with skin detachment. SJS is characterized by mucous membrane erosions and blisters on less than 10% of the total body surface area, whereas TEN involves more than 30% of the total body surface area.58 The more severe the reaction, the more likely it is that it was drug-induced. Cases of severe cutaneous ADRs to lamotrigine (e.g., SJS and TEN) have been reported.59 The prevalence of severe cutaneous ADRs associated with lamotrigine has been reported to be as high as one in 1,000 in adults and is higher in children. The risk is increased in the presence of valproic acid.
Complete blood counts, liver enzyme measurements, and chest x-rays should be performed to rule out concurrent internal organ involvement.
Treatment of EM, SJS, and TEN includes discontinuance of a suspected drug and such supportive measures as careful wound care, hydration, and nutritional support.60 The use of corticosteroids in SJS and TEN is controversial.61 Intravenous immunoglobulin (IVIg, 0.4 to 1.0 g/kg/day for 2 to 4 days), which contains naturally occurring Fas ligand (FasL)-blocking antibodies, has been shown in most reports to halt progression of TEN, especially when IVIg is started early.62-64 Patients who have developed a severe cutaneous ADR (EM, SJS, or TEN) should not be rechallenged with the drug or undergo desensiti-zation with the medication.
Figure 7 Coumarin-induced skin necrosis in a 57-year-old woman who was given coumarin as treatment for atrial fibrillation.
Pustular Eruptions
Simple eruptions Acneiform Eruptions
Eruptions morphologically mimicking acne vulgaris may be associated with drug ingestion. Iodides, bromides, adrenocorti-cotropic hormone, corticosteroids, isoniazid, androgens, lithium, dactinomycin, and phenytoin are reported to induce acnelike lesions.65 Acne fulminans was induced by testosterone in 1% to 2% of adolescent boys who were treated for excessively tall stature.66
Drug-induced acne often appears on the face and back, but it may appear in atypical areas, such as arms and legs, and is usually monomorphous. Comedones are usually absent. Fever is absent. Acneiform eruptions do not affect prepubertal children, indicating that previous hormonal priming is a prerequisite. Topical tretinoin may be useful when the drug cannot be stopped.
Complex eruptions
Acute Generalized Exanthematous Pustulosis
Acute generalized exanthematous pustulosis is characterized by acute onset, fever, and a cutaneous eruption with non-follicular sterile pustules on an edematous erythema, generally starting within days of drug ingestion67 [see Figure 6]; leukocytosis is another common finding. Generalized desquamation occurs 2 weeks later. Differential diagnosis includes pustular psoriasis, subcorneal pustular dermatosis (Sneddon-Wilkinson disease), hypersensitivity syndrome reaction with pustulation, and pustular eruptions of infancy.
Table 2 Clinical Pearls to Identify Anticoagulant-Induced Skin Necrosis
|
Interval to Onset |
Location |
Other |
|
|
Coumarin-induced skin necrosis |
3-5 days |
Adipose-rich sites |
— |
|
Heparin-induced thrombocytopenia and thrombosis |
4-14 days |
Extremities |
Thrombocytopenia occurs concurrently |
|
Purple-toe syndrome |
3-8 wk |
Acral location |
Often occurs after angiography |
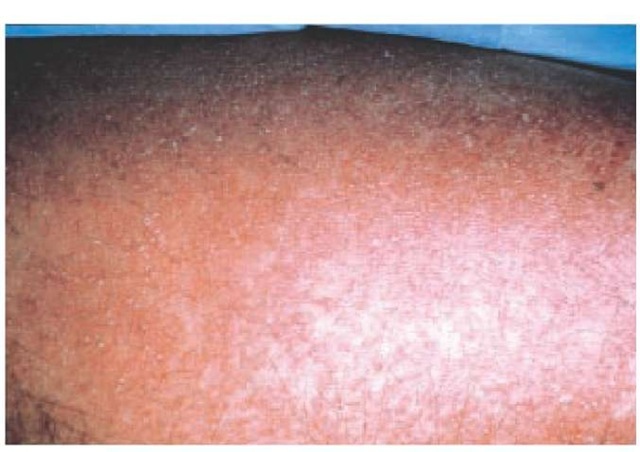
Figure 8 Leukocytoclastic vasculitis developed in this 47-year-old woman taking hydrochlorothiazide.
Acute generalized exanthematous pustulosis is most commonly associated with P-lactam and macrolide antibiotic usage. Many other drugs have been implicated, however, including calcium channel blockers and analgesics. The estimated incidence rate is approximately one to five cases per million per year.68 Discontinuance of therapy is usually the extent of treatment necessary in most patients, although some patients may require the use of corticosteroids. Patch testing to the putative drug is often positive, resulting in a localized pustular reaction.
Other Eruptions
Anticoagulant-induced skin necrosis
Anticoagulant drugs may induce hypercoagulable states with subsequent vascular infarction and cutaneous necrosis [see Figure 7]. Both coumarin and heparin can induce skin necrosis. Clinical pearls that can help differentiate these reactions are the location, timing, platelet count, and primary diagnosis [see Table 2].
The pathogenesis of coumarin-induced skin necrosis is the paradoxical development of occlusive thrombi in cutaneous and subcutaneous venules caused by a transient hypercoagulable state. This condition results from the suppression of the natural anticoagulant protein C at a greater rate than natural procoagu-lant factors. Coumarin-induced skin necrosis is associated with protein C and protein S deficiency, but pretreatment screening is not warranted. An association with a heterozygote for the factor V Leiden mutation has been recently reported.69
It is estimated that one in 10,000 persons who take coumarin are at risk for this adverse event.70 The prevalence is four times higher in women than in men. In both sexes, the peak incidence occurs in the sixth and seventh decades of life. Afflicted patients tend to be obese.
Coumarin-induced skin necrosis begins 3 to 5 days after initiation of treatment. Painful red plaques develop in adipose-rich sites such as breasts, buttocks, and hips. These plaques may blister, ulcerate, or develop into necrotic areas. An accompanying infection, such as pneumonia, viral infection, or erysipelas, may occur in as many as 25% of patients. Purple-toe syndrome occurs 3 to 8 weeks after initiation of coumarin therapy.
Treatment entails the discontinuance of coumarin, administration of vitamin K, and infusion of heparin at therapeutic doses. Fresh frozen plasma and purified protein C concentrates have been used.71 Supportive measures for the skin are recommended. Plastic surgery for remediation is necessary in 60% of affected patients.
Drug-induced lichenoid eruptions
Drug-induced lichen planus produces lesions that are clinically and histologically indistinguishable from those of idio-pathic lichen planus. Many drugs, including beta blockers, penicillamine, NSAIDs, gold, and ACE inhibitors, especially captopril, have been reported to produce this reaction.
The latent period between the start of administration of the drug and appearance of the eruption is variable. The mean latent period is between 2 months and 3 years for penicillamine, approximately 1 year for beta-adrenergic blocking agents, and 3 to 6 months for ACE inhibitors. The latent period may be shorter if the patient was previously exposed to the drug.72 In general, resolution usually occurs within 2 to 4 months.
Rechallenge with the culprit drug has been attempted in a few patients, with reactivation of symptoms within 4 to 15 days.73 Patch testing has not proved helpful in most cases of drug-induced lichen planus. However, results of patch tests performed with contact inducers of lichen drug eruptions (e.g., color-film developers and dental restorative materials) are usually positive.72
Drug-induced vasculitis
Drug-induced vasculitis represents approximately 10% of the acute cutaneous vasculitides and usually affects small vessels [see Figure 8].74 Drug-induced vasculitis should be considered in any patient with small vessel vasculitis that is usually confined to the skin.75 Drugs that are most frequently associated with vasculitis include propylthiouracil, hydralazine, gran-ulocyte colony-stimulating factor (G-CSF), granulocyte-macro-phage CSF (GM-CSF), allopurinol, cefaclor, minocycline, peni-cillamine, phenytoin, and isotretinoin.73 The average interval to onset of drug-induced vasculitis is 7 to 21 days.76
The clinical hallmark of cutaneous vasculitis is palpable pur-pura, classically found on the lower extremities, although any cutaneous site may be affected. Urticaria can be a manifestation of small vessel vasculitis. Unlike nonvasculitic allergic urticaria, vasculitic urticaria lasts longer than 1 day, may evolve into pur-puric lesions, and may be accompanied by hypocomplemen-temia.77 Other features are hemorrhagic bullae, urticaria, ulcers, nodules, Raynaud disease, and digital necrosis. The same vas-culitic process may also affect internal organs, such as the liver, kidney, gut, and CNS, and is potentially life threatening.
Histologically, the small blood vessels of the dermis display fibrinoid necrosis, polymorphonuclear infiltration into the blood vessel wall, extravasation of red blood cells, and nuclear dust. Direct immunofluorescence may show deposits of IgM and C3 in the blood vessel walls. Therefore, these reactions are immune complex-dependent drug reactions. The immune complexes may be composed of antibodies directed against drug-related haptens, but this has not been proved.
Drug-induced vasculitis can be difficult to diagnose, and diagnosis is often one of exclusion.78 Alternative causes of cuta-neous vasculitis, such as infection or autoimmune disease, must be eliminated.
Treatment consists of drug withdrawal.Therapy for patients with severe manifestations includes hemodialysis, pulse corti-costeroids, cyclophosphamide, and plasmapheresis.73