Clinical Manifestations
As with other types of diffuse infiltrative lung disease, dyspnea on exertion and cough are the most prominent symptoms. Physical examination typically reveals fine, dry inspiratory rales. Cyanosis may be noted when hypoxemia is severe, and features of cor pulmonale (e.g., elevated jugular venous pressure, edema, and a prominent pulmonic second heart sound) indicate advanced disease. Clubbing of the digits is noted in 25% to 50% of cases but is often absent early in the course of the disease. There are no other extrathoracic manifestations of disease. Clinical evidence of extrapulmonary disease (e.g., arthritis, skin disease, or serositis) suggests a systemic disorder, such as sarcoido-sis or one of the collagen vascular diseases, rather than IPF.
Diagnosis
A diagnosis of IPF is established by correlating histopatho-logic and clinical findings. Clinically, IPF is suspected when a diffuse infiltrative lung disease occurs with no involvement of other organ systems and there is no apparent relation to infection, environmental exposure, or drugs. The chest radiograph shows no evidence of hilar adenopathy or pleural effusion. Laboratory tests in IPF are generally unrevealing, except for the presence of a positive antinuclear antibody or positive rheumatoid factor in up to 50% of cases. Thus, serum antinuclear antibody and rheumatoid factor cannot be relied on to differentiate IPF from diffuse infiltrative lung disease associated with collagen vascular disorders if the titers are low.
The initial approach to diagnosing IPF is to exclude as many causes of diffuse infiltrative lung disease as possible by a combination of history and clinical examination. However, a number of causes remain that cannot be reliably identified without a lung biopsy. Such causes include stage III sarcoidosis, lymphan-gitic carcinomatosis, lymphangioleiomyomatosis, eosinophilic granuloma, DIP/RBILD, NSIP/F, LIP, AIP, and the more diffuse forms of BOOP.
Imaging studies The radiographic features are nonspecific and most often consist of a bilateral reticular or reticulonodular pattern that typically appears in the lower lung fields; if cor pulmonale develops, enlargement of the pulmonary arteries and hypertrophy of the right ventricle occurs. In as many as 10% of patients with symptomatic IPF, the chest radiograph may be entirely normal. Radiographic findings in patients with IPF are limited to the lung fields. Hilar lymphadenopathy or pleural effusion suggests a different cause of diffuse infiltrative lung disease.
HRCT may be helpful at this stage of the evaluation because the pattern of abnormalities seen in IPF is specific, and many of these other causes have characteristic and very different pat-terns.6 Also, HRCT is likely to reveal abnormalities in cases in which the chest radiograph is normal.
The HRCT pattern in IPF includes patchy, peripheral, sub-pleural, bibasal reticular abnormalities with minimal ground-glass opacity. With advanced disease, honeycomb lung and traction bronchiectasis/bronchiolectasis, indicating end-stage lung fibrosis, are seen. Extensive areas of ground-glass haziness, indicating an acinar filling process, are not characteristic of IPF but may be seen in AIP, LIP, DIP/RBILD, and NSIP/F.
Pulmonary function testing The physiologic abnormalities of IPF are basically the same as those described for diffuse infil-trative lung disease. The classic composite physiologic picture of IPF is reduced DLCO, restriction of lung volume, exercise-induced oxygen desaturation, and absence of airflow obstruction. Some of the alterations correlate with the pathologic findings.
Histopathologic features The histopathologic features of IPF are nonspecific. Similar histopathologic findings are observed in diffuse infiltrative lung disease associated with collagen vascular disease, various types of drug-induced diffuse disease, chronic hypersensitivity pneumonitis, asbestosis, and other disorders. Chronic fibrosis and a scant inflammatory cell infiltrate, the essential pathologic features of IPF, are common reactions to a variety of agents.
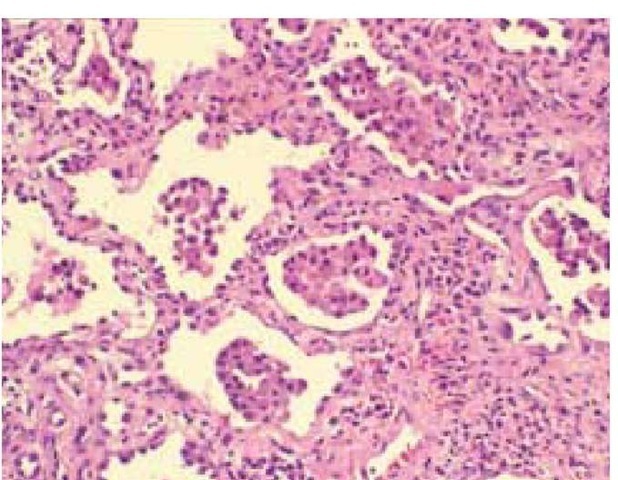
The histopathologic picture in IPF is termed usual interstitial pneumonitis (UIP) and is characterized by minimal interstitial inflammatory round cell infiltrate, widening of alveolar septa, and fibrosis with fibroblastic foci43 [see Figure 8]. The distribution of the lesion is irregular: areas of intense fibrosis can coexist with areas of near-normal lung in the same open lung biopsy specimen. In some cases, more than one histologic pattern is seen; in such cases, if any areas of UIP are seen, the clinical diagnosis should be IPF.
UIP must be differentiated from DIP/RBILD, AIP, LIP, NSIP/F, and BOOP, because of different prognoses 43 and treatment approaches (see below).
Lung biopsy Transbronchial lung biopsy is a sensitive tool for diagnosing sarcoidosis and lymphangitic cancer and may reveal pathologic features suggestive of IPF (i.e., fibrosis and widened alveolar septa with scant inflammatory cell infiltrate). Open lung biopsy is needed to exclude other causes of diffuse disease. It is up to the clinician to decide whether to proceed to open lung biopsy when clinical evaluation and transbronchial lung biopsy are highly suggestive of IPF. Open lung biopsy is advisable when there is any doubt about whether there is an infectious cause. Open lung biopsy should usually be performed in younger patients to establish the diagnosis of the underlying disorder with a reasonable degree of certainty.5 Older patients with typical clinical features and a compatible trans-bronchial lung biopsy result may reasonably be spared the morbidity associated with open lung biopsy. In certain cases, it may be reasonable to diagnose IPF without any tissue biopsy.
For example, a tissue biopsy may not be needed to diagnose IPF in an elderly patient who has had progressive dyspnea on exertion for many months to years and who has fine, end-in-spiratory crackles and clubbing of the digits on examination, bilateral coarse interstitial infiltrates, low-titer serum antinu-clear antibody, and no evidence of an environmental or a drug-related disorder.52
Prognosis and Treatment
The median survival for patients with newly diagnosed IPF is 2 to 3 years. Most deaths result from progressive pulmonary impairment. Adverse prognostic factors include cigarette smoking, severe dyspnea, low lung compliance, and high numbers of fibroblastic foci in the lung biopsy specimen.53 Patients with IPF are also at increased risk for carcinoma of the lung.
Corticosteroids have previously been used in the initial management of IPF. However, reviews of the lung biopsies of the patients in the studies supporting steroid use have suggested that the 20% to 30% of patients responding to steroids probably did not have UIP by the current pathologic criteria.55
Cyclophosphamide and azathioprine have been used in IPF, often in conjunction with prednisone or in patients in whom prednisone failed. The rationale for the use of these agents is similar to that for the use of steroids. A recent prospective trial found that cyclophosphamide had limited efficacy in a group of IPF patients in whom steroid therapy had failed or had produced unacceptable side effects.56 Only one study provides evidence that azathioprine has any significant efficacy.57 Current opinion holds that these drugs are more toxic than beneficial in IPF.42 Colchicine, a drug thought to have antifibrotic properties, was not found to affect survival in one study.58 Single-lung transplantation has been used successfully for end-stage lung fibrosis.59 Patients who are potential transplantation candidates and whose DLCO has dropped below 40% should be referred to a transplant center.60
Alternative therapies for IPF have been proposed.61 A trial of interferon beta was unsuccessful. In a randomized controlled trial, a combination of interferon gamma-1b and low-dose prednisolone given to IPF patients whose condition was unresponsive to steroids or other immunosuppressive agents led to greater improvement in lung function over 12 months than was seen with prednisolone alone.62 A larger randomized trial of interferon gamma-1b is under way.
Desquamative interstitial pneumonia/respiratory bronchiolitis interstitial lung disease
Patients with DIP/RBILD present with cough and dyspnea of insidious onset.43,63 Clubbing of digits is present in about 50% of cases. The average age at onset of symptoms is the early to middle 40s, which is significantly younger than the age at onset of IPF symptoms. Men are affected twice as often as women. Almost all of these patients are cigarette smokers, suggesting that the disease may be caused or initiated by tobacco use.
The chest radiograph typically shows vague, bibasilar opacities that correlate with ground-glass densities on HRCT. Some studies have found more reticulonodular and linear changes.43,63 The lung biopsy specimen in DIP/RBILD is characterized by a fairly homogeneous pattern in which alveolar spaces are filled with pigmented alveolar macrophages. These accumulations of macrophages are often accentuated in peribronchiolar air spaces, sparing the more distal air spaces [see Figure 9].43,63 Discontinuance of smoking has been associated with improvement. Many patients improve without therapy other than smoking cessation. Steroids have been associated with a beneficial response in about 60%. The mortality is 20% to 30%, and the mean survival is 12 years.
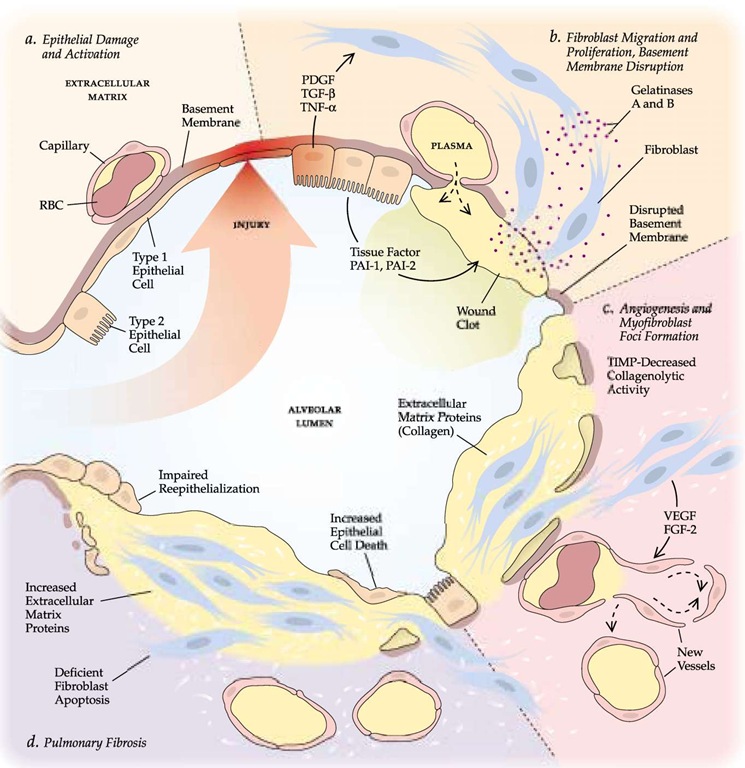
Figure 7 The current hypothesis is that idiopathic pulmonary fibrosis (IPF) originates with repeated episodes of injury to the epithelium that result in formation of a microscopic lesion—in essence, a wound—in the wall of the alveolus (a). A fibrin clot then develops in the alveolar space, and the alveolar cells secrete growth factors (PDGF, TGF-p, TNF-a) that induce migration and differentiation of fibroblasts; in turn, the fibroblasts produce gelatinases that further disrupt the normal extracellular matrix and the basement membrane (6). Angiogenic factors (VEGF, FGF-2) induce new vessel formation. Small foci of myofibroblasts appear (c). An imbalance between increased synthesis of extracellular matrix proteins, mainly collagen, and decreased collagenolytic activity results in the progressive deposition of matrix, and deficient apoptosis of fibroblasts and increased epithelial cell death impair reepithelialization. The result of this aberrant healing process is pulmonary fibrosis (d). (FGF—fibroblast growth factor; MMP—matrix metalloproteinase; PAI—plasminogen activator inhibitor; PDGF—platelet-derived growth factor; TGF—transforming growth factor; TIMP—tissue inhibitor of metalloproteinase; TNF— tumor necrosis factor; VEGF—vascular endothelial growth factor)
Acute interstitial pneumonia
Acute interstitial pneumonia is a distinct idiopathic condition that produces respiratory failure of rapid onset.43,64 AIP is also known as Hamman-Rich syndrome.
Figure 8 Usual interstitial pneumonia is characterized by alveolar septal thickening, caused by fibrosis; minimal inflammatory cell infiltrate; honeycombing; and irregular involvement from one region to the next.
The clinical presentation of AIP is similar to that of ARDS except that, in patients with AIP, no predisposing factor can be identified. A viral prodrome often precedes onset. Over a period of less than 30 days, a symptom complex of dyspnea, cough with mucoid sputum, and fever evolves into respiratory failure. Imaging studies by routine chest radiograph or HRCT show diffuse pulmonary infiltrates with ground-glass changes and consolidation.
The diagnosis is made by the recognition of a clinical illness compatible with ARDS in the absence of a predisposing factor after the exclusion of other alveolar-filling diseases, such as infectious pneumonia (especially PCP), alveolar hemorrhage, and eosinophilic pneumonia. The diagnosis is usually made through bronchoscopy with bronchoalveolar lavage.
AIP is characterized pathologically by the findings of diffuse alveolar damage. It cannot be differentiated from ARDS caused by sepsis, toxins, or shock [see Figure 10]. AIP evolves through the same sequence of pathologic patterns as ARDS [see 14:X Pulmonary Edema].
Treatment is largely supportive, through use of oxygen with either noninvasive or invasive mechanical ventilation. Steroids are advocated, but their use is not supported by controlled trials.
The mean 6-month mortality is 33% to 78%. Survivors may have recurrences or develop chronic progressive interstitial lung disease.
Lymphocytic interstitial pneumonia
LIP is an interstitial lung disease characterized by diffuse or localized lymphocytic infiltration of the alveolar and interstitial areas of the lung.43,65 This disorder can occur in association with a number of autoimmune processes (especially Sjogren syndrome), dysproteinemias, immunodeficiency (AIDS and common variable immunodeficiency), drug reactions (e.g., to phenytoin), or bone marrow transplantation; or it can occur as an idiopathic process.
LIP occurs more often in women than in men. The mean age at onset is 56 years. Patients present with dyspnea and cough, but the associated disorder may dominate the clinical picture. Rales and lymphadenopathy are common physical findings.
Imaging studies typically show reticulonodular infiltrates. The presence of hilar or mediastinal adenopathy or pleural effusion suggests lymphoma. HRCT will usually show a mixture of interstitial and alveolar (ground-glass) changes. Thin-walled cysts are often present.
Bronchoscopic specimens can be useful, showing a striking lymphocytosis (mostly B cells) on BAL and revealing lymphocytic infiltration on transbronchial lung biopsy [see Figure 11], The pathologist will often need to use special studies to differentiate LIP from neoplastic forms of lymphocytic infiltration of the lung.
Patients with LIP should be treated for any underlying disorder. Some patients respond to steroids, though many require immunosuppressive agents.
Nonspecific interstitial pneumonia/fibrosis
NSIP/F has often been confused with IPF. In retrospective analyses of patients who had been diagnosed with IPF. many were found to have NSIP/F and were noted to have a better prognosis than patients with IPF.43
NSIP/F occurs in middle-aged adults, with a mean age at onset of 49 years, but it can also affect children and older adults. There is a slight female predominance. Although some cases are idiopathic, many patients have collagen vascular diseases or immunodeficiency (including HIV infection), and some have a history of environmental or therapeutic drug exposures. A few patients have a history of an acute lung injury, such as that caused by pneumonia, ARDS, or surgery.
Dyspnea, cough, and sometimes fever are the dominant clinical features, with imaging studies showing bilateral interstitial infiltrates. HRCT scanning demonstrates bilateral, patchy areas of ground-glass abnormality, but this pattern is also seen with other disorders and therefore does not permit the confident diagnosis of NSIP/F.
NSIP/F can be diagnosed only by lung biopsy. As with IPF, transbronchial lung biopsy is not likely to produce an adequate specimen, so open biopsy is required. Pathologically, NSIP/F is characterized by a temporally uniform mixture of alveolar wall inflammation (mostly lymphocytes and plasma cells) and fibrosis [see Figure 12].
Figure 9 Desquamative interstitial pneumonia/respiratory bronchiolitis interstitial lung disease. The alveoli and septal walls are filled with macrophages, and no fibrosis is apparent.
Figure 10 Acute interstital pneumonia. The biopsy specimen shows diffuse alveolar damage.
Steroids appear to provide improvement in more than half of the patients with NSIP/F, and the prognosis for patients with NSIP/F is significantly better than that for patients with IPF.66 Some patients experience relapses, and a minority have a progressive illness despite treatment.