The menstrual cycle is orchestrated through the interaction of the hypothalamus, pituitary gland, ovaries, and uterus. The hypothalamus secretes gonadotropin-releasing hormone (GnRH), which stimulates the pituitary to secrete luteinizing hormone (LH) and follicle-stimulating hormone (FSH). LH and FSH stimulate follicular growth, ovulation, corpus luteum formation, and the secretion of estradiol and progesterone by the ovaries, which leads to the cyclic growth and shedding of the uterine endometrium. At the endometrial level, the menstrual cycle has three key phases: estradiol stimulates endometrial growth, progesterone induces differentiation of the endometri-um, and withdrawal of estradiol and progesterone results in sloughing of the endometrium and menstrual bleeding.
Failure of any part of this process can result in the absence of menstruation. A girl may not start to menstruate when she reaches puberty (primary amenorrhea); or as is far more common, a woman who has been menstruating may have her cycles cease (secondary amenorrhea).
Pathophysiology
The hypothalamus contains approximately 10,000 GnRH-se-creting neurons that drive the menstrual cycle by secreting pulses of GnRH. The embryonic precursors of the GnRH neurons develop in the olfactory bulb and migrate to the arcuate and preop-tic nuclei. Improper development of the olfactory bulb in early embryogenesis can result in both anosmia and amenorrhea because of the absence of the GnRH neurons (Kallman syndrome).
The main function of the GnRH neurons is to receive neural signals from the brain and transform them into an endocrine output, the pulsatile release of GnRH. This conversion of electrical signal into endocrine output takes place in the arcuate nucleus. To determine the appropriate pulse frequency and amplitude of GnRH secretion, the hypothalamus monitors numerous environmental cues, including body composition, stress, nutritional status, and emotion. From a teleologic perspective, it is inefficient to ovulate and reproduce if the environment is hostile to the nurturing of a newborn.
The hypothalamus is the conductor that sets the tempo for the menstrual cycle. When the hypothalamus secretes GnRH at a low pulse frequency and amplitude, the pituitary gland is not driven to secrete LH and FSH, so the ovary and endometrium become quiescent. This causes amenorrhea. When the hypo-thalamus secretes GnRH at an abnormally elevated pulse frequency and amplitude, there is an exaggerated secretion of LH, causing the ovary to become androgenic and secrete testosterone. Follicular growth is blocked and no ovulation occurs. This results in the polycystic ovary syndrome (PCOS), which can be associated with oligomenorrhea or amenorrhea.
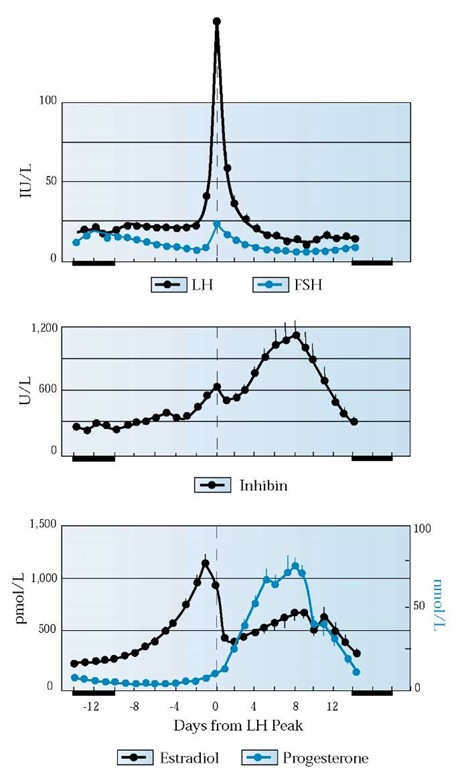
The pituitary gland is the main link between the brain and ovarian function. Secretion of the gonadotropins LH and FSH by the pituitary gland is not only stimulated by GnRH from the hy-pothalamus but also modulated by the negative feedback of steroid and protein hormones from the ovaries, especially estradiol, progesterone, and inhibin A and B [see Figure I]. Estradiol and inhibin A are secreted by growing follicles and the corpus lu-teum. Inhibin B is secreted by the small antral follicles and growing follicles. Progestersone is secreted by the corpus luteum.
The follicle is the functional unit of the ovary. At puberty, the ovary contains approximately 300,000 follicles, of which only a few hundred will be ovulated in the woman’s lifetime. The follicle has three components: an outer shell of thecal cells that respond to LH and secrete the androgen precursor an-drostenedione; an inner cell mass of granulosa cells that respond to FSH by converting androstenedione to estradiol; and, at the center of the follicle, the oocyte [see Figure 2]. Resting follicles are recruited into a cohort of active follicles, only one of which will be destined to ovulate each cycle; the remainder undergo atresia.
Figure 1 Interaction between pituitary and ovarian hormones. The surge of luteinizing hormone (LH) and follicle-stimulating hormone (FSH), which prompts ovulation, is followed by an increase in inhibin and progesterone secretion and a decrease in estradiol.
Under FSH stimulation, granulosa cell numbers increase dramatically, from approximately 10 cells in the primordial follicle to approximately 50 million cells in the preovu-latory dominant follicle. The dominant follicle, which is the one destined to ovulate, can be identified early in its development by three characteristics: it has captured large amounts of FSH in its follicular fluid, it has the optimal number of granulosa cells for its size, and it produces much more estradiol than testosterone. Excess stimulation by LH results in an androgen-dominant follicle, which is characterized by thecal cell overac-tivity and a preference to produce testosterone over estradiol. In PCOS, the ovary contains many androgen-dominant follicles that do not have the potential to ovulate.
The purpose of the menstrual cycle is to generate a single oocyte for fertilization and to prepare the endometrium for implantation. Estradiol stimulates endometrial proliferation, gland formation, and vascular growth in the endometrium.
Figure 2 Relationship between granulosa cells and thecal cells of the ovarian follicle. Luteinizing hormone (LH) stimulates thecal production of androstenedione, which diffuses to the granulosa cells. In the granulosa cells, follicle-stimulating hormone (FSH) stimulates the conversion of androstenedione to estradiol.
When stimulated by estradiol, the endometrium increases production of its own intracellular estrogen receptors, which augment its response to estradiol; it also produces more progesterone receptors. After ovulation, progesterone causes gland development and differentiation of the endometrium; in addition, glycogen is stored in preparation for embryo implantation. If pregnancy does not occur, the decline in ovarian production of estradiol and progesterone causes vasospasm of the endometrial blood vessels and sloughing of the endometrium, resulting in menstrual bleeding.
Abnormalities in GnRH secretion, LH or FSH secretion, ovarian follicular function, or endometrial function can cause amen-orrhea or oligomenorrhea. In a given patient, the differential di-agnosis—and hence the evaluation—depends on whether the amenorrhea is secondary or primary.
Secondary Amenorrhea
Secondary, or adult-onset, amenorrhea is present when a woman who had been menstruating has no menses for longer than three of her previous cycles, or 6 months. Determining the cause of secondary amenorrhea starts with measuring serum levels of human chorionic gonadotropin (hCG), prolactin, FSH, and testosterone and calculating the body mass index (BMI) [see Figure 3].
The most common cause of secondary amenorrhea is pregnancy. Pregnancy is best diagnosed by measuring the serum or urine hCG level. The hCG pregnancy test is one of the most accurate in medicine, with a sensitivity and specificity exceeding 99%.
In women who are not pregnant, the most common causes of secondary amenorrhea are as follows: hypothalamic dysfunction (low GnRH pulse frequency, amplitude, or both), pituitary dysfunction (low LH and FSH production). loss of all ovarian follicles (ovarian failure). PCOS. Asherman syndrome (intrauterine adhesions), and thyroid disease [see Table I].1
Amenorrhea caused by hypothalamic dysfunction
Low GnRH Secretion
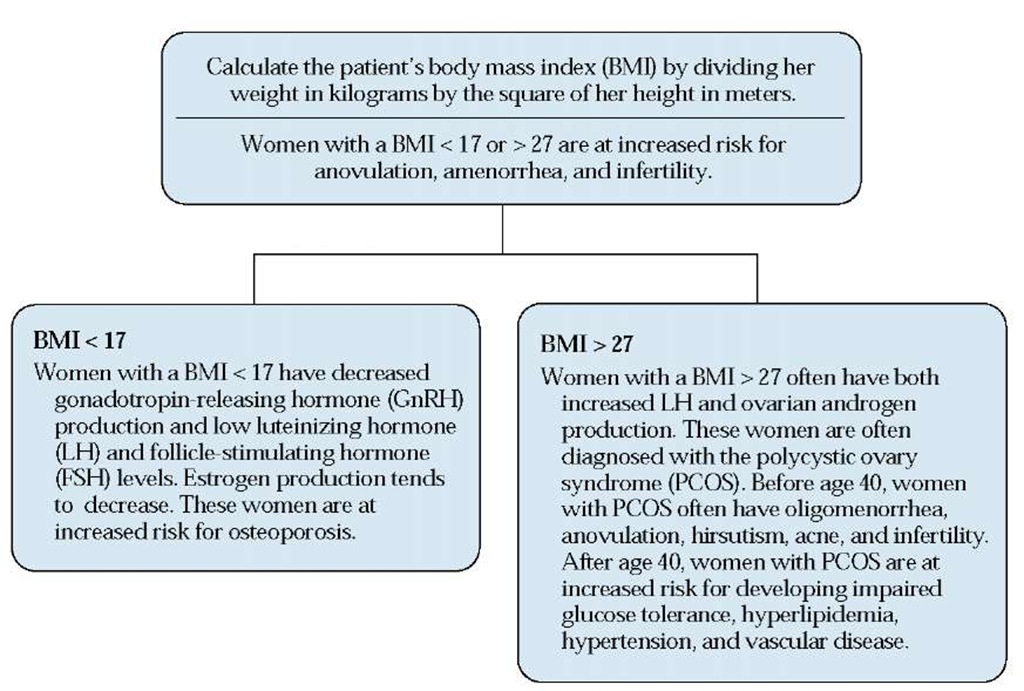
Low BMI, vigorous exercise, psychosocial stress, and nutritional abnormalities decrease GnRH production. This reduces LH and FSH secretion and can cause amenorrhea.
Diagnosis The patient typically has a history of regular vigorous exercise, psychosocial stress, or reduced caloric intake. On physical examination, the BMI is often less than 20 kg/m2 [see Figure 4]. Serum FSH, prolactin, and testosterone levels are usually reported as normal in women with secondary amenorrhea caused by low GnRH secretion. Women with secondary amenorrhea from hypothalamic hypofunction should be screened for eating disorders; the prevalence of eating disorders in this population is 5% to 10%. Rarely, hypothal-amic dysfunction can be caused by structural abnormalities of the hypothalamus, including lymphoma, histiocytosis X, sar-coidosis, and hypothalamic cysts.
The absence of menses in association with hypothalamic hy-pofunction suggests severe estrogen deficiency, although varying degrees of hypoestrogenism may be present. The severity of the hypoestrogenism can be tested by performing a progestin withdrawal test [see Figure 5].2 Serum estradiol assays are not sufficiently accurate for this purpose. Women with amenorrhea resulting from hypothalamic hypofunction often have triiodothyronine (T3) levels less than 70 ng/dl, reverse T3 levels greater than 40 ng/dl (similar to those of nutritionally deprived individuals), and elevated levels of cortisol secretion (as seen in depressed women or women under significant stress).
Figure 3 Diagnosis and treatment of secondary amenorrhea. (|-hCG—|-human chorionic gonadotropin; FSH—follicle-stimulating hormone)
Treatment Reversing the underlying cause of hypothala-mic hypofunction (reducing psychosocial stress, gaining weight, lowering exercise intensity) often results in resumption of ovulatory menses. However, many women with amenor-rhea from low GnRH secretion prefer to maintain the exercise and nutritional regimens that cause the amenorrhea, and those with an eating disorder may not respond to treatment of it. These women are best treated with hormone replacement. Therapeutic choices include oral contraceptives and low-dose hormone replacement, such as a standard combined continuous regimen of conjugated estrogens, 0.625 mg, plus medroxyprogesterone acetate, 2.5 mg, daily. Cyclic hormone replacement may also be used. The use of sustained-release vaginal gel to supply the progesterone component of hormone replacement therapy has been reported.3 Vitamin D (400 IU/day) and calcium (1,200 to 1,500 mg/day) supplements should be administered to slow the rate of decline in bone mineral density associated with low estrogen levels.
Amenorrhea caused by pituitary dysfunction
The most common pituitary diseases that cause secondary amenorrhea are prolactin-secreting pituitary tumors (prolactin-omas), the empty sella syndrome, Sheehan syndrome, and other pituitary tumors, such as those that secrete adrenocorti-cotropic hormone (ACTH) or growth hormone.
Prolactin-Secreting Pituitary Tumors
Most pituitary tumors are monoclonal, which indicates that they arise from a somatic mutation in a single progenitor cell. In general, pituitary tumors are benign and slow growing.
Diagnosis The most common causes of an elevation in the serum prolactin level are, in order of frequency, advanced pregnancy; the use of psychotropic medications that are dopamine antagonists, such as haloperidol; prolactin-secreting pituitary tumors; hypothyroidism; and renal failure. After excluding those causes, the workup should focus on the pituitary.
Table 1 The Most Common Causes of Secondary Amenorrhea in Women Who Are Not Pregnant
|
Organ |
Cause |
Relative Frequency (%) |
|
Hypothalamus |
Abnormalities of height/weight and nutrition |
15 |
|
Exercise |
10 |
|
|
Psychosocial stress |
10 |
|
|
Infiltrative disease or tumors of the hypothalamus (sarcoidosis, histiocytosis, craniopharyngioma) |
< 0.1 |
|
|
Pituitary |
Prolactin-secreting pituitary tumor |
17 |
|
Empty sella syndrome |
1 |
|
|
Sheehan syndrome |
< 1 |
|
|
ACTH-secreting tumor (Cushing disease) |
< 1 |
|
|
GH-secreting tumor |
< 1 |
|
|
Ovary |
Premature ovarian failure |
10 |
|
Polycystic ovary syndrome |
30 |
|
|
Uterus |
Asherman syndrome (intrauterine synechiae) |
5 |
|
Other |
Nonclassical adrenal hyperplasia |
< 1 |
|
Thyroid disease |
1 |
|
|
Ovarian tumors |
< 1 |
ACTH—adrenocorticotropic hormone
GH—growth hormone
A magnetic resonance imaging scan of the hypothalamus and pituitary can confirm the diagnosis of prolactinoma. The MRI is also used to determine whether the diameter of the tumor is less than 10 mm (microprolactinoma) or greater than 10 mm (macroprolactinoma), because this measurement has clinical implications. Finally, the MRI can assess for possible involvement of the sella turcica and the optic chiasm.
If the MRI shows a pituitary tumor, it is important to also measure serum insulinlike growth factor-1 (IGF-1). IGF-1 levels will be elevated in patients whose pituitary tumor secretes growth hormone; this is a more reliable test than measurement of growth hormone itself.
Treatment In general, microprolactinomas have a benign course and can be managed by the patient’s primary care physician. Observational studies indicate that over a period of 4 to 6 years, 95% of microprolactinomas do not increase in size.4,5 Macroprolactinomas, however, can be associated with significant complications, such as pituitary apoplexy and compression of the optic chiasm, and should be managed by an endocrinolo-gist. The initial treatment of both microprolactinomas and macroprolactinomas should be medical therapy, not surgery.
The two best approaches to management of microprolactin-omas in women with amenorrhea are low-dose oral contraceptives and a dopamine agonist (bromocriptine, pergolide, or cabergoline). Both contraceptives and dopamine agonists can initiate regular withdrawal bleeding and prevent osteoporosis. In women with microprolactinomas, treatment with an estro-gen-progestin oral contraceptive is safe and is not associated with clinically significant tumor growth.
Women with amenorrhea caused by a prolactinoma who wish to become pregnant should receive treatment with a dopamine agonist to induce ovulation. Dopamine agonists directly suppress prolactin production by the tumor and cause an increase in endogenous GnRH secretion, which stimulates pituitary secretion of LH and FSH and consequently induces follicle development and ovulation. In addition, dopamine agonists decrease the size of prolactin-secreting pituitary tumors.
Bromocriptine has been used to induce ovulation in women with hyperprolactinemia for more than 25 years.9 In one study of 280 women with hyperprolactinemia, bromocriptine normalized the circulating prolactin level in 82% of the women.10 The main side effects associated with the use of bromocriptine are nausea, vomiting, and orthostatic hypotension. To minimize these potential side effects, it is recommended that bromocriptine be initiated at a dose of 1.25 mg at bedtime. After 1 week, the dosage can be increased to 1.25 mg twice daily. The dosage can then be increased to 2.5 mg twice dally, a standard dosage that successfully reduces serum prolactin in most women with hyperprolactinemia.10 Long-acting oral and in-jectable forms of bromocriptine have been developed,11,12 but those are not yet available in the United States.
Pergolide, an ergot dopamine agonist, has been approved by the Food and Drug Administration for the treatment of Parkinson disease but not for the treatment of hyperprolactinemia. Unlike bromocriptine, pergolide can be given once a day. Per-golide is the least expensive of the dopamine agonists; its cost is about one sixth that of cabergoline.
Cabergoline is a non-ergot dopamine agonist that is administered once or twice a week and causes less nausea than bromocriptine or pergolide.13 The FDA-approved dosage of cabergoline is 0.25 mg twice a week. Many clinicians start cabergoline at a dosage of 0.5 mg a week, then increase the dosage to 1 mg once or twice a week, depending on the response of the serum prolactin level (see below). In about 25% of women, the serum prolactin level returns to normal through therapy with cabergoline at a dosage of 1 mg a week; in these patients, the dosage can be reduced to 0.5 mg a week and the serum prolactin level will remain normal. About one half of women who do not respond to bromocriptine treatment will respond to treatment with cabergoline.14 Many authorities believe that cabergoline is more effective than bromocriptine in treating hyperprolactinemia.15 In a series of 459 women with hyperprolactinemia and amenorrhea, 83% of the women treated with cabergoline experienced normalization of their pro-lactin levels, compared with 52% of those treated with bromocriptine.16 Cabergoline is significantly more expensive than bromocriptine, however.
With dopamine agonist therapy, near-maximal decreases in serum prolactin levels are typically achieved after 4 weeks of treatment. Serum prolactin levels should be measured approximately 1 month after initiating therapy and about 1 month after a change in dose or drug. If the serum prolactin concentration is normal and no side effects have occurred, the initial dose should be continued. If serum prolactin has not decreased to normal and no side effects are present, the dose should be gradually increased. Maximal dosages of the dopamine agonists are as follows: bromocriptine, 5 mg twice daily; pergolide, 0.25 mg once daily; and cabergoline, 1.5 mg two or three times weekly. If the serum prolactin level does not decrease to normal, switching to a different dopamine agonist may be effective. If the patient cannot tolerate the side effects of the dopamine agonist initially prescribed, a different dopamine agonist may be tried. If the patient experiences side effects with all the dopamine agonists, then vaginal administration of bromocriptine can be tried.17 If all attempts at medical therapy fail, transsphenoidal surgery is indicated. Successful removal of the tumor will result in the normalization of the prolactin secretion level and resumption of ovulatory menses.
Figure 4 Relation of body mass index to anovulation.
After correction of hyperprolactinemia, about 80% of women will ovulate; cumulative pregnancy rates of 80% are commonly observed.18 Treatment is usually discontinued once a pregnancy is diagnosed. However, in women with a macroprolactinoma, therapy should be continued throughout pregnancy to reduce the risk that the tumor will grow and cause neurosurgical complications, such as compression of the optic nerve.